ISO 13485 Certification in NEOM
Get Free Consultation
Safety and quality are essential in the medical devices industry, which is why ISO 13485 was created. With stricter rules at every stage of a product’s life—from design to delivery—companies are increasingly required to demonstrate their quality management processes and adopt best practices in everything they do.
ISO 13485 Certification in NEOM for QMS in Medical Device Manufacturing get expert advice from the Best ISO 13485 Consultants in Jeddah with Popularcert. In Neom, as regulations continue to tighten, businesses in this field must consistently show their commitment to quality and make these practices a standard part of their operations to maintain ISO 13485 Certification in Neom.
What is ISO 13485 and why is it important?
ISO 1385 is a Global Quality Management standard. It’s put out by the International Organization for Standardization, much like ISO 9001. This standard is specific to medical devices. It takes into account all the needs of those who manufacture, supply, distribute, or have a role in the medical device field.
Goal: To have strong and efficient procedures for marketing medical gear globally, this shows using the ISO 13485 system meets many legal needs at once. To show they’ve complied with the rules, the ISO 1385 fs verified by an independent certifying group.
ISO 13485 is a recognized standard in Neom. It means that a business has passed an audit done by a certification body after passing, they get a certificate.
ISO 13485 Certification – Getting certified can help boost trust. It shows that your product or service lives up to what your customers expect. In some fields, you’re legally or contractually bound to be certified.
This certificate typically lasts from 3 to 5 years each year, an audit occurs to make sure that high quality management continues.
How to Get ISO 13485 Certification In Neom?
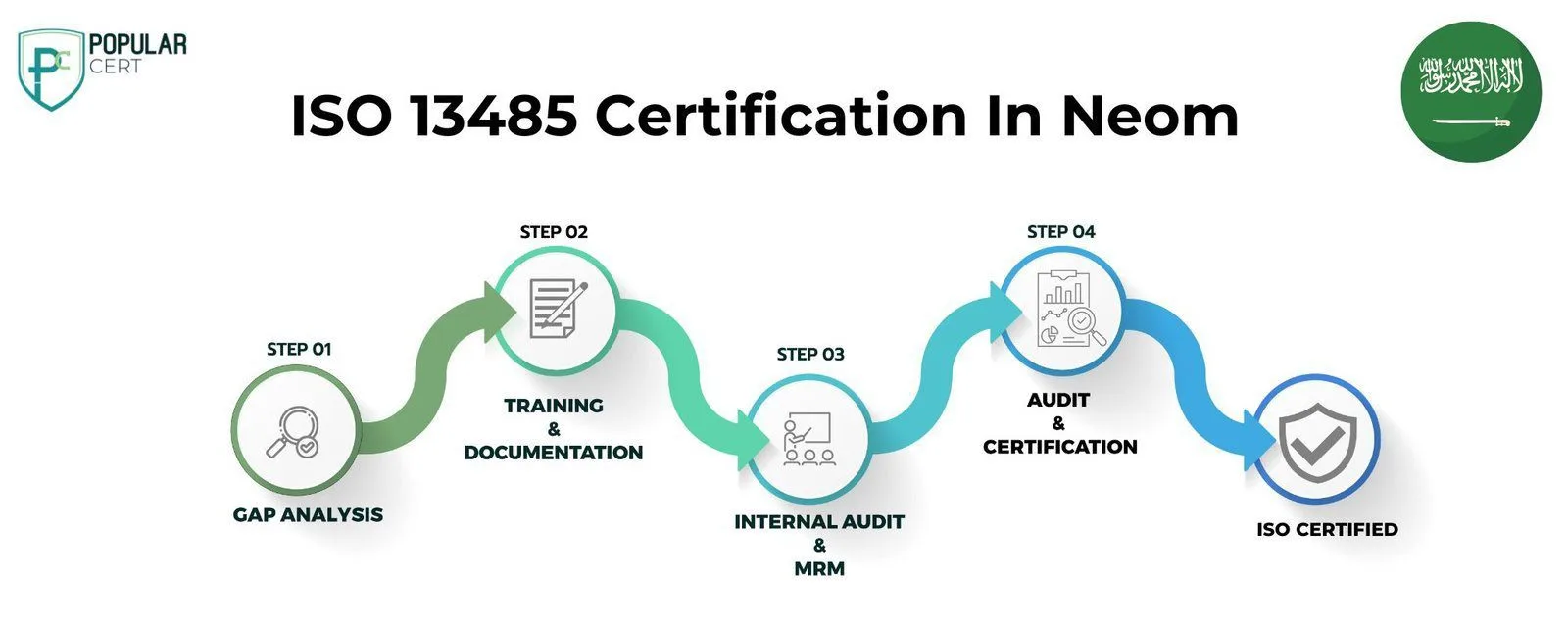
Process to Get ISO 13485 Certification In Neom
Consultation and Gap Analysis
PopularCert’s experts assess your organization’s unique requirements and current systems in Neom. We conduct a detailed gap analysis to identify areas requiring improvement to align with ISO standards.
Planning, Documentation, and Policy Development
Based on the gap analysis findings, we develop a tailored implementation plan, define resource requirements, and support the creation of essential policies and documentation, ensuring seamless integration into your existing organizational structure.
Training and Awareness
We provide comprehensive training to ensure your team fully understands ISO requirements and their roles in effectively maintaining the management system.
Internal Audit and Management Review
Our specialists conduct internal audits to assess system performance and address any non-conformities. A management review ensures the system aligns with your organization’s objectives and ISO standards.
External Certification Audit and Certification
Upon successfully completing the external audit conducted by the certification body, your organization in Neom will be awarded the ISO 13485 certificate. This certification highlights your commitment to quality management specific to medical devices, continuous improvement, and regulatory compliance. It demonstrates your dedication to meeting international standards while enhancing credibility, ensuring product safety, and building customer trust.
Benefits of ISO 13485 Certification In Neom
- Regulatory Compliance: Ensures your medical devices meet international regulatory requirements, facilitating market access.
- Improved Product Quality: Enhances the quality of medical devices through a structured quality management system.
- Risk Management: Helps identify and control risks associated with medical device design, production, and distribution.
- Enhanced Customer Trust: Demonstrates commitment to product safety and quality, boosting customer confidence.
- Operational Efficiency: Streamlines processes, reduces errors, and improves overall productivity.
- Global Market Access: Certification aligns with global standards, making it easier to expand into international markets.
Types Of ISO Certification In Neom
Get Free Consultation
Our Clients


















Cost of ISO 13485 Certification In Neom
The cost of ISO 13485 certification in Neom can vary depending on several factors, including the size of your organization, the complexity of your medical device processes, and the current state of your quality management system. Working with experienced consultants like Popularcert can help you streamline the process and reduce costs by offering expert guidance tailored to your business needs. Investing in ISO 13485 certification not only ensures compliance with global medical device standards but also improves product quality, operational efficiency, and customer trust.
ISO 13485 Certification in NEOM for QMS in Medical Device Manufacturing. Get expert advice on how to get and how to apply for ISO 13485 certification from the best consultants in Jeddah with Popularcert.
Why Choose PopularCert For ISO 13485 Certification in Neom ?
PopularCert is the ideal choice for ISO 13485 certification in Neom due to its expert team of consultants who specialize in medical device quality management systems. With personalized guidance, PopularCert ensures a smooth certification process, from gap analysis to final audit preparation. Their cost-effective services are tailored to meet your business needs while ensuring full compliance with international standards. Choosing PopularCert means prioritizing product safety, regulatory compliance, and customer satisfaction, helping your organization achieve long-term success in the medical device industry.
GET A FREE CONSULTATION NOW
FAQ
What is ISO 13485 certification?
ISO 13485 is an internationally recognized standard for Quality Management Systems (QMS) in the medical device industry. It ensures compliance with regulatory requirements and enhances product safety and quality.
Why is ISO 13485 certification important for medical device manufacturers in Neom?
ISO 13485 certification helps medical device manufacturers meet global quality standards, improve operational efficiency, ensure product safety, and gain market credibility.
How can I get ISO 13485 certification in Neom?
To obtain ISO 13485 certification in Neom:
- Conduct a gap analysis.
- Develop and implement the QMS.
- Perform internal audits and management reviews.
How do I apply for ISO 13485 certification in Neom?
You can apply for ISO 13485 certification by:
- Contacting Popularcert for expert consultancy.
- Submitting your QMS documentation.
- Scheduling an audit with a certification body.
How much does ISO 13485 certification cost in Neom?
The cost varies depending on the organization’s size, complexity, and existing QMS. Contact Popularcert for a tailored quote.
