ISO 13485 Certification In Erbil
Get Free Consultation
ISO 13485 Certification in Erbil helps medical device manufacturers and related organizations demonstrate their commitment to quality, safety, and regulatory compliance. By aligning with international ISO 13485 standards, companies in Erbil can ensure consistent product quality, meet local and global regulatory requirements, and improve patient safety. This certification not only enhances operational efficiency but also builds customer trust and opens access to international markets. Achieving ISO 13485 strengthens a company’s credibility and positions it as a reliable player in the competitive medical device industry.
What is ISO 13485 Certification?
An organization’s ISO 13485 certification demonstrates its commitment to standardized quality assurance in the design, manufacturing, and servicing of medical devices. This certification ensures consistency, enhanced efficiency, regulatory compliance, and improved patient and customer satisfaction—key goals for any business operating in the healthcare sector. To become certified, a company must undergo a thorough compliance audit by an accredited third party, verifying adherence to the stringent requirements of ISO 13485, established by the International Organization for Standardization (ISO).
Why ISO 13485 Certification is Important in Erbil
ISO 13485 certification is especially vital in Erbil, a growing industrial hub in Iraq, where medical device manufacturing and healthcare services are rising. This certification helps companies meet local and international regulatory requirements, improving operational efficiency and product quality. For businesses in Erbil, ISO 13485 is a strategic tool that ensures safer products, builds trust with regulatory authorities and clients, and enhances competitiveness in both regional and global markets. It also enables easier access to international supply chains and healthcare markets. By obtaining ISO 13485 certification, medical device companies in Erbil can elevate their brand reputation, drive innovation, and ensure long-term business sustainability.
How to Get ISO 13485 Certification in Erbil?
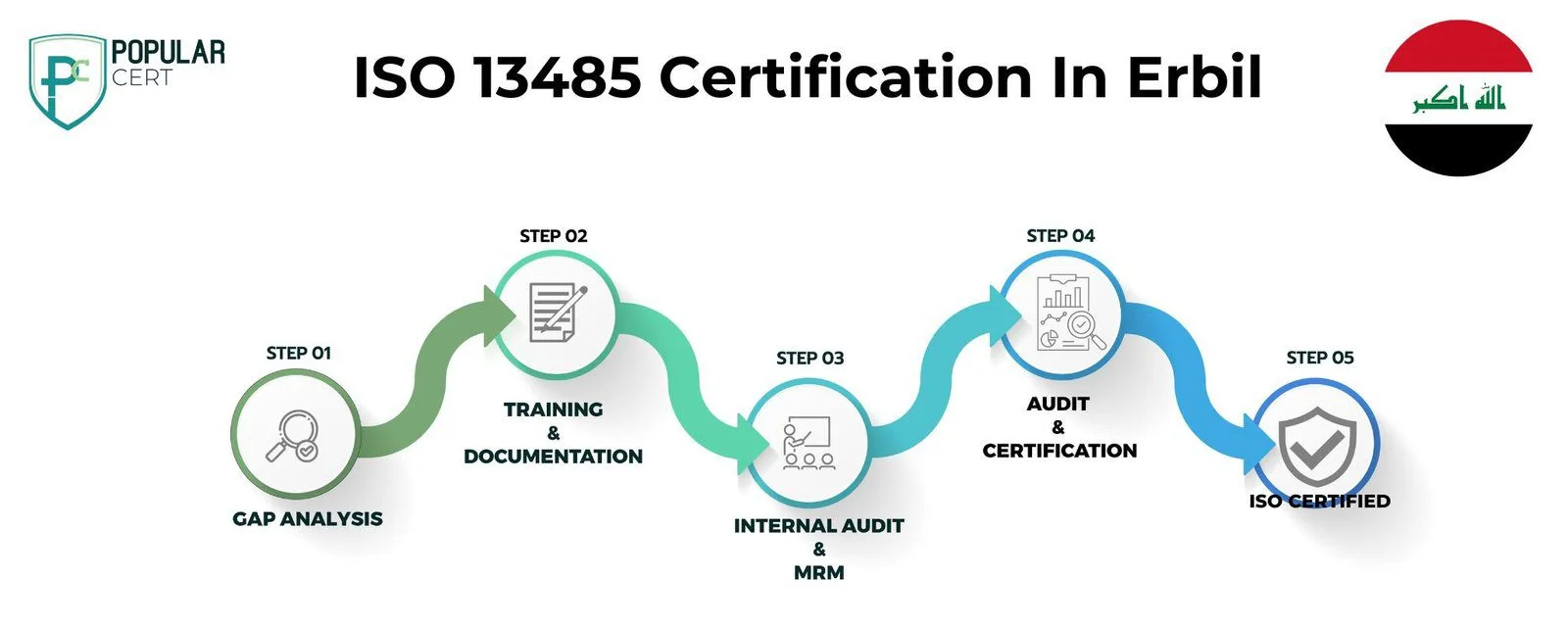
Process to Get ISO 13485 Certification In Erbil
Consultation and Gap Analysis
PopularCert’s experts begin by understanding your organization’s needs and current management practices in Erbil. We then conduct a gap analysis to identify areas requiring improvement to achieve ISO 13485 certification, ensuring your organization is well-prepared to meet international standards for medical device quality management. This comprehensive approach guarantees that your business is on the right path to enhance product quality, ensure patient safety, and comply with global regulatory requirements, ensuring long-term success and growth in the medical device industry.
Planning, Documentation, and Policy Development
Based on the gap analysis, we develop a detailed implementation plan for your organization in Erbil, allocate resources, and assist in creating essential policies and documentation required for ISO 13485 certification. These policies and procedures are seamlessly integrated into your existing framework, ensuring compliance with regulatory requirements and effective implementation of the Quality Management System (QMS) for medical devices. This tailored approach ensures a smooth transition towards achieving certification while enhancing product quality, patient safety, and operational efficiency.
Training and Awareness
We provide comprehensive training for your staff in Erbil, ensuring they understand the requirements of ISO 13485 certification and their role in effectively implementing and maintaining the Quality Management System (QMS) for medical devices. Our training programs are tailored to meet the specific needs of your organization, empowering your team to successfully achieve and sustain certification. This approach ensures that your staff is equipped with the knowledge and skills needed for continuous improvement, product quality, patient safety, and compliance with international medical device standards.
Internal Audit and Management Review
After implementing the ISO 13485 Quality Management System (QMS) for medical devices, we conduct an internal audit in Erbil to assess its efficiency and identify any non-conformities. Following this, a management review is carried out to ensure the system aligns with your organization's goals and regulatory compliance requirements, ensuring readiness for certification. This process ensures that your organization is fully prepared and compliant, setting the stage for a successful external audit and ISO 13485 certification.
External Certification Audit and Certification
After successfully completing the external audit by the certification body, your organization in Erbil will be awarded ISO 13485 certification. This certification highlights your commitment to high standards of quality management in the medical device industry and continuous improvement. It demonstrates your dedication to excellence, enhances your credibility, and builds customer trust, especially in competitive markets. Achieving ISO 13485 certification in Erbil helps position your organization as a leader in medical device manufacturing, boosting your reputation and opening doors to new opportunities for growth.
Benefits of ISO 13485 Certification in Erbil
- Enhanced Product Quality : Ensures the delivery of safe, effective, and high-quality medical devices.
- Regulatory Compliance : Meets international standards and regulations like the EU Medical Device Regulation (MDR).
- Increased Market Access :Facilitates entry into global markets by meeting international requirements.
- Customer Trust :Builds confidence among clients and healthcare professionals by demonstrating a commitment to quality.
- Operational Efficiency :Streamlines processes, reduces waste, and improves overall efficiency.
- Risk Management : Identifies and mitigates risks associated with medical device production and usage.
- Competitive Advantage :Distinguishes your organization in a competitive market, attracting more customers.
- Continuous Improvement :Promotes a culture of ongoing quality enhancement and compliance.
Types Of ISO Certification In Erbil
Get Free Consultation
Our Clients


















Which Industries Need ISO 13485 Certification in Erbil?
ISO 13485 is important for any company that works with medical devices. In Erbil, the following industries can benefit from getting this certification:
- Medical Device Manufacturers: Companies that make tools like surgical instruments, implants, or diagnostic machines.
- Pharmaceutical Companies: Businesses that make or work with drug delivery systems or health-related products.
- Hospitals and Clinics: Healthcare providers that use or manage medical devices.
- Medical Equipment Suppliers: Companies that sell or distribute medical tools and devices.
- Contract Manufacturers: Factories that make medical products for other companies.
- Sterilization and Packaging Services: Businesses that clean and pack medical items safely.
Cost of ISO 13485 Certification in Erbil
The cost of ISO 13485 certification in Erbil varies based on factors such as your business size, the complexity of your medical device manufacturing processes, and the scope of the certification. The process includes assessing your current quality management practices, implementing necessary improvements, and undergoing audits by an accredited certification body. While the initial investment may seem high, ISO 13485 certification offers long-term benefits such as improved product quality, enhanced patient safety, reduced risks of non-compliance, and increased customer trust. The cost may also depend on whether you engage external consultants for guidance or manage the process internally.
Why Choose PopularCert For ISO 13485 Certification in Erbil?
PopularCert is a globally renowned consulting company specializing in certification, advisory, and auditing services. We are the trusted choice for organizations seeking ISO 13485 certification in Erbil due to our experienced, ethical consultants and proven success record. For ISO 13485 certification in Erbil, choose PopularCert, a leader in consultancy, certification, and auditing services.
GET A FREE CONSULTATION NOW
FAQ
What is ISO 13485?
ISO 13485 is an international standard that outlines the requirements for a quality management system (QMS) for medical device manufacturing and related services.
What are the Benefits of ISO 13485 certification in Erbil?
ISO 13485 certification in Erbil ensures regulatory compliance, enhances product quality, and opens doors to international markets by demonstrating a commitment to safety and quality in medical device manufacturing.
Who Should Get ISO 13485 Certification in Erbil?
Erbil’s med-tech designers and producers, even their installers and maintainers, should chase after the ISO 13485 certificate. Medical companies of all sorts need this. Getting certified is key. It helps convince customers about product safety, meet official rules and enter overseas markets who prefer certified partners. Certifying with the ISO 13485 can help firms run smoother, get more done and show they are serious about quality. This matters in the worldwide health rivalries.
How Does ISO 13485 Certification Work in Erbil?
ISO 13485 certification in Erbil follows a structured process to ensure medical device manufacturers meet the highest quality standards.



















