GMP Certification in ZAMBIA
Get Free Consultation
PopularCert delivers reliable GMP (Good Manufacturing Practices) certification services in Zambia, helping manufacturers maintain consistent product quality and safety throughout production. GMP certification in Zambia supports businesses in key regions such as Lusaka, Ndola, and Kitwe in adhering to hygiene, documentation, and process control requirements. Our approach aligns with international GMP guidelines and can be complemented by standards like ISO 9001 (Quality Management) and ISO 22000 (Food Safety Management), ensuring your operations meet both regulatory expectations and global market demands.
What Is GMP certification?
GMP (Good Manufacturing Practices) certification ensures that products are consistently produced and controlled according to quality standards. It focuses on hygiene, process control, and safety in manufacturing environments. GMP helps minimize risks in production, especially in pharmaceuticals and food industries, ensuring products are safe for consumption and meet international quality requirements for market approval and trust.
Why Is GMP Certification Important In Zambia?
- In Zambia’s growing pharmaceutical and food sectors, GMP certification plays a vital role in ensuring that products are safe, high-quality, and consistently manufactured. As consumers become more conscious of health standards, businesses that follow GMP earn greater trust and improve their ability to meet regulatory requirements both locally and for export.
- Having GMP certification in Zambia isn’t just about compliance; it’s a business advantage. It reduces production errors, enhances operational efficiency, and helps brands stand out in competitive markets. For manufacturers looking to expand beyond borders or secure government contracts, GMP shows a clear commitment to excellence and product safety, giving them a strong edge in the industry.
How to Get GMP Certification In Zambia?
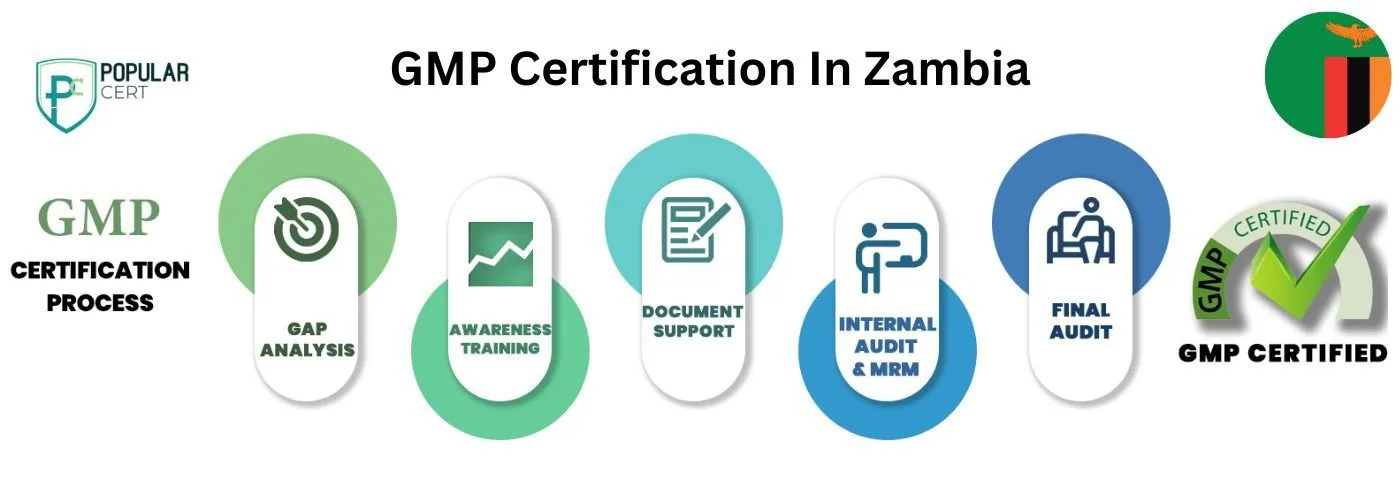
Process to Get GMP Certification In Zambia
Consultation and Gap Analysis
Begin with a gap analysis to assess current practices against GMP requirements. Identify areas needing improvement and create an action plan for compliance. This helps set a clear path toward certification.
Develop and Document GMP Systems
Develop or update your quality management systems to meet GMP standards. This includes creating comprehensive documentation on processes, procedures, and safety protocols that align with regulatory requirements.
Training and Implementation
Train employees on GMP practices, ensuring they understand the importance of quality, hygiene, and safety. Implement these practices across all stages of production, focusing on maintaining high standards in manufacturing operations.
Internal Audit
Conduct internal audits to assess the effectiveness of your GMP system. Address any gaps or non-conformities before the external certification audit.
External Certification Audit
Engage an accredited certification body to conduct the external audit. After successful evaluation of your GMP system, you will receive certification, ensuring compliance and product safety.
Benefits of GMP Certification in Zambia
- Improved Product Quality: GMP ensures the consistent production of high-quality, safe products by enforcing strict manufacturing processes, reducing defects and contamination risks.
- Regulatory Compliance: Certification ensures compliance with local and international regulations, minimizing legal risks and avoiding penalties or recalls. It also helps businesses stay ahead of changing industry standards.
- Enhanced Reputation: Achieving GMP Certification boosts a company's reputation for quality and safety, increasing trust among consumers, partners, and regulatory bodies.
- Global Market Access: GMP Certification opens doors to international markets, as many countries require GMP compliance for product importation. It enhances business competitiveness globally.
- Operational Efficiency: GMP practices streamline production processes, reduce waste, and improve resource management, leading to cost savings and more efficient operations.
- Consumer Confidence: Certification reassures customers that products are manufactured under strict hygiene and safety standards, increasing consumer satisfaction and loyalty.
Types Of ISO Certifications In Zambia
- ISO Certification in Zambia
- ISO 9001 Certification
- ISO 14001 Certification
- ISO 45001 Certification
- ISO 22000 Certification
- ISO 27001 Certification
- ISO 17025 Certification
- ISO 13485 Certification
- CE Mark Certification
- ISO 20000-1 Certification
- GMP Certification
- Halal Certification
- SOC-1 Certification
- SOC-2 Certification
Get Free Consultation
Our Clients


















Cost of GMP Certification in Zambia
The cost of GMP certification in Zambia varies based on the type of product, size of your facility, and the current level of regulatory compliance. Whether you’re a manufacturer, packager, or supplier, working with experienced GMP consultants in Zambia can help reduce costs and streamline implementation.
Key factors affecting the cost:
- Nature of the product (food, pharma, cosmetics, etc.)
- Size and number of production units
- Existing quality management systems
- Staff training and internal audit needs
- Documentation and SOP preparation requirements
- Certification body’s audit fees
- Follow-up audit and compliance checks
For tailored advice, request a free GMP consultation in Zambia from the best GMP certification company in Zambia to receive accurate cost estimates.
Why Choose PopularCert For GMP Certification in Zambia?
PopularCert is your trusted partner for GMP certification in Zambia, helping businesses meet global manufacturing and safety standards. Our experienced consultants guide you through every step of the GMP certification process, ensuring your operations align with Zambia’s regulatory requirements and international best practices. Whether you’re a pharmaceutical manufacturer or in food production, we tailor our approach to fit your industry.
Why choose PopularCert in Zambia:
- Expert support for GMP certification Zambia
- Customized GMP compliance consulting Zambia
- Affordable GMP certification Zambia services
- Smooth documentation and audit readiness
- Recognized among trusted GMP consultants in Zambia
Get your manufacturing processes certified with confidence. PopularCert simplifies the path to GMP success in Zambia.
GET A FREE CONSULTATION NOW
FAQ
What is GMP certification in Zambia and why is it important?
GMP (Good Manufacturing Practices) certification in Zambia ensures that food, pharmaceutical, and cosmetic products are consistently produced and controlled according to international safety and quality standards. It’s important for businesses that want to gain consumer trust, meet regulatory needs, and expand into regional or global markets.
Who should apply for GMP certification in Zambia?
Manufacturers of food products, medicines, cosmetics, and dietary supplements in Zambia should consider GMP certification. It’s especially valuable for businesses aiming to prove their commitment to hygiene, safety, and product consistency.
How much does GMP certification cost in Zambia?
The cost of GMP certification in Zambia depends on the size of your facility, the type of products you manufacture, and your current systems. PopularCert offers tailored and affordable GMP solutions for businesses across Zambia.
