ISO 13485 certification in BASRA
Get Free Consultation
PopularCert offers specialized ISO 13485 certification services in Basra, supporting medical device manufacturers in meeting international quality and regulatory standards. ISO 13485 certification in Basra helps companies in districts like Al Ashar, Al-Jubaila, and Al Maqal implement robust Quality Management Systems tailored to the healthcare sector. Our services are based on the ISO 13485 standard and can be effectively combined with related frameworks such as ISO 14971 (Risk Management for Medical Devices) and ISO 9001 (Quality Management), ensuring product reliability, compliance, and global market readiness.
What Is ISO 13485 Certification?
ISO 13485 certification is a globally recognized standard for quality management systems in the medical device industry. It ensures that companies consistently meet regulatory and customer requirements for the design, production, and distribution of medical devices. This certification demonstrates a company’s commitment to product safety, risk management, and ongoing quality improvement in healthcare-related manufacturing.
Why is ISO 13485 Certification Important in Basra?
- In Basra’s growing medical and healthcare sectors, ISO 13485 certification plays a vital role in establishing trust. It assures that companies involved in manufacturing or supplying medical devices follow strict international standards for quality and safety. This is especially important as healthcare providers and regulators in Iraq increasingly demand reliable, compliant, and safe medical products.
- By achieving ISO 13485 certification, businesses in Basra gain a competitive edge in both local and international markets. It helps reduce product recalls, improve operational efficiency, and build stronger relationships with hospitals and distributors. In a region aiming to modernize its healthcare infrastructure, certified companies show they are ready to support that vision with professionalism and quality-driven practices.
How to Get ISO 13485 Certification in Basra?
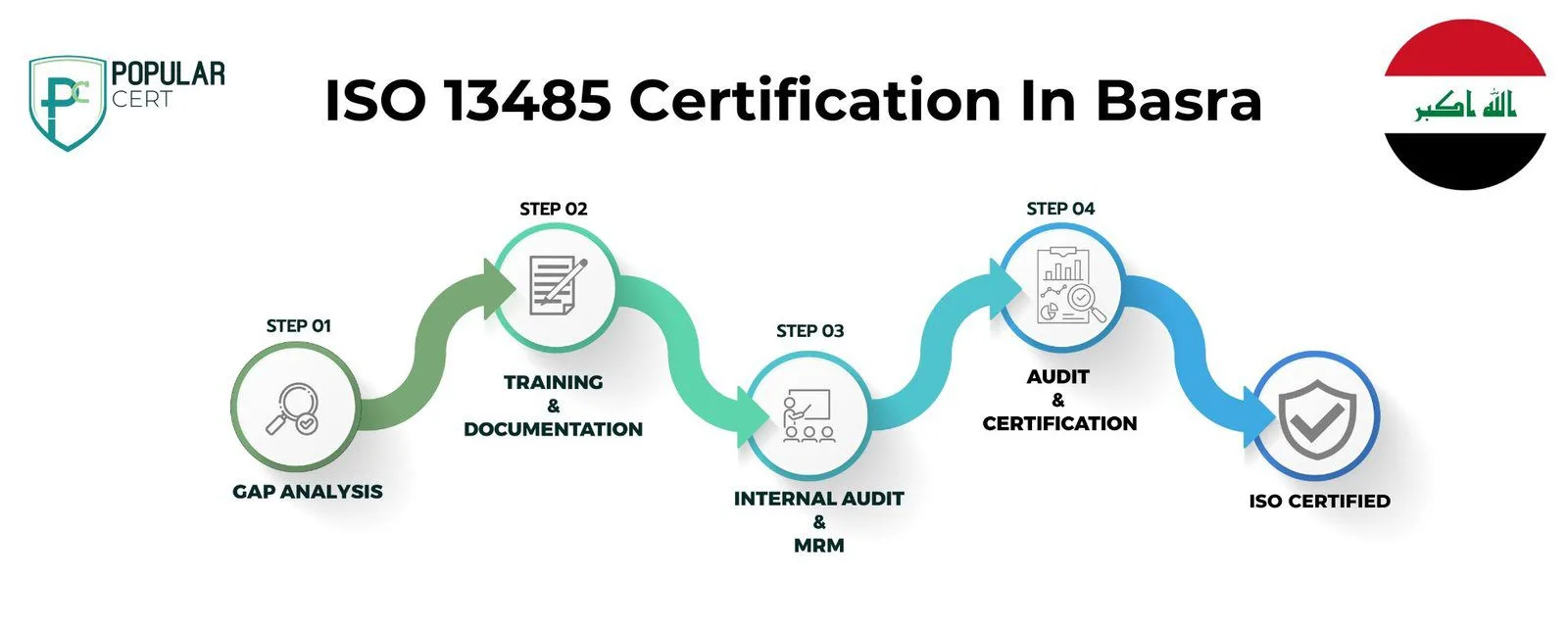
Process to Get ISO 13485 Certification In Basra
Consultation and Gap Analysis
PopularCert’s experts begin by understanding your organization’s needs and current management practices. We then conduct a gap analysis to identify areas requiring improvement to achieve ISO 13485 certification in Basra, ensuring your organization is well-prepared to meet international standards for medical devices and regulatory compliance.
Planning, Documentation, and Policy Development
Based on the gap analysis, we develop a detailed implementation plan, allocate resources, and assist in creating essential policies and documentation required for ISO 13485 certification. These policies and procedures are seamlessly integrated into your existing framework, ensuring compliance with medical device regulations and effective implementation in Basra.
Training and Awareness
We provide comprehensive training for your staff, ensuring they understand the requirements of ISO 13485 certification and their role in effectively implementing and maintaining the quality management system for medical devices. Our training programs are tailored to meet the specific needs of organizations in Basra, empowering your team to successfully achieve and sustain ISO 13485 certification.
Internal Audit and Management Review
After implementing the ISO 13485 management system, we conduct an internal audit to assess its efficiency and identify any non-conformities related to the quality management system for medical devices. Following this, a management review is carried out to ensure the system aligns with your organization's goals and compliance requirements in Basra, ensuring readiness for ISO 13485 certification.
External Certification Audit and Certification
After successfully completing the external audit by the certification body, your organization will be awarded ISO 13485 certification. This certification highlights your commitment to high standards of quality management for medical devices and continuous improvement. It demonstrates your dedication to excellence, enhances your credibility, and builds customer trust, especially for organizations in Basra.
Benefits of ISO 13485 Certification in Basra
- Regulatory Compliance : Getting the ISO 13485 certification means meeting the rules set by various important groups. These include the Iraqi National Regulatory Authority and global ones like the FDA and EMA.
- Enhanced Quality Management :Implementation of ISO 13485 ensures the formation of strong quality management systems, leading to improved product safety and quality.
- Market Access : Getting the ISO 13485 certification can help your business. It proves you meet international quality standards. This can make entering global markets easier.
- Customer Confidence : Having a certificate builds trust. Customers and rule makers believe in the company more. This means they trust what the company sells and does more.
- Competitive Advantage :Earning the ISO 13485 badge sets a company apart. It attracts possible clients who value top notch standards and adherence to rules.
- Operational Efficiency : With ISO 13485, your operations get an upgrade. Standard processes mean fewer mistakes and less wasted effort.
- Risk Management : ISO 13485 puts a lot of focus on managing risk. It does this all through the product's life from start to finish. This helps organizations spot risks and deal with them well.
- Continuous Improvement : Being certified brings a habit of always pushing for better. This leads to increased results, customers satisfaction and a strong organization..
Types Of ISO Certification In Basra
Get Free Consultation
Our Clients


















Cost of ISO 13485 Certification in Basra
The cost of ISO 13485 certification in Basra depends on factors like company size, product range, and the current state of your quality management system. For those searching ISO 13485 consultants in Basra or trying to estimate the certification cost in Basra, understanding what influences the price is key.
Main cost factors include:
- Scope of medical device manufacturing or services
- Number of employees and departments involved
- Customization needs for documentation
- Internal staff training requirements
- Audit duration and certification body charges
- Gap analysis and QMS readiness
- Post-certification surveillance fees
Many manufacturers begin with a free ISO 13485 consultation in Basra to get an accurate estimate from the best ISO certification company in Basra.
Why Choose PopularCert For ISO 13485 Certification in Basra?
PopularCert offers specialized support for ISO 13485 certification in Basra, helping medical device companies meet international quality and regulatory standards. Our experienced ISO consultants in Basra guide you through every step of the ISO 13485 certification process, ensuring a smooth, efficient, and cost-effective journey. We understand local industry needs and align them with global requirements for maximum impact.
Why businesses in Basra trust PopularCert:
- Tailored ISO 13485 solutions for Basra’s healthcare industry
- Trusted ISO consultants in Basra with industry expertise
- Smooth and clear ISO certification process in Basra
- Focus on regulatory compliance and risk management
- Competitive pricing for ISO 13485 certification in Basra
Build quality-driven healthcare operations with PopularCert by your side.
GET A FREE CONSULTATION NOW
FAQ
What is ISO 13485 certification in Basra and why is it needed?
ISO 13485 certification in Basra is a globally recognized quality standard for medical device companies. It’s essential for businesses that want to ensure product safety, meet Iraq’s regulatory expectations, and gain access to international healthcare markets.
Who should get ISO 13485 certified in Basra?
Medical device manufacturers, suppliers, and distributors in Basra should consider ISO 13485 certification. It ensures quality management practices that improve safety, reduce risk, and increase global market acceptance.
How much does ISO 13485 certification cost in Basra?
The cost of ISO 13485 certification in Basra depends on your business size, processes, and current quality controls. PopularCert offers tailored and affordable ISO 13485 solutions to help Basra-based companies meet certification requirements with ease.
