ISO 13485 certification in Kenya
Get Free Consultation
ISO 13485 Certification in Kenya is essential for organizations involved in the design, production, installation, and servicing of medical devices. This internationally recognized standard ensures that companies implement a robust quality management system tailored to the medical device industry. By achieving ISO 13485 certification, Kenyan businesses demonstrate their commitment to product safety, regulatory compliance, and consistent quality. The certification process involves a rigorous audit by an accredited body, verifying that the organization meets both local and global regulatory requirements. ISO 13485 boosts credibility with healthcare providers and regulators, enhances patient safety, improves operational efficiency, and opens doors to international markets—making it a strategic asset for medical device companies in Kenya.
What is ISO 13485?
ISO 13485 is a global standard specifically for medical devices, ensuring that manufacturers, suppliers, and distributors follow strict quality control procedures. It’s similar to ISO 9001 but focuses on the medical device industry. This standard helps businesses show they have solid processes in place for designing, making, and selling medical devices safely and reliably across the world. Many countries now require medical device manufacturers to have an ISO 13485-compliant Quality Management System to meet regulatory standards. Certification of this system by an accredited body proves that companies are following the necessary regulations and quality standards.
Why ISO 13485 Certification is Important in Kenya?
ISO 13485 Certification is important in Kenya because it helps businesses in the medical device industry meet global quality standards, ensuring their products are safe, reliable, and compliant with regulations. It not only boosts the safety and quality of medical devices but also improves operational processes, builds customer trust, and helps companies stay competitive in the rapidly evolving medical industry. By getting ISO 13485 certified, businesses can ensure they meet both local and international requirements, giving them an edge in the market.
How to Get ISO 13485 Certification In Kenya?
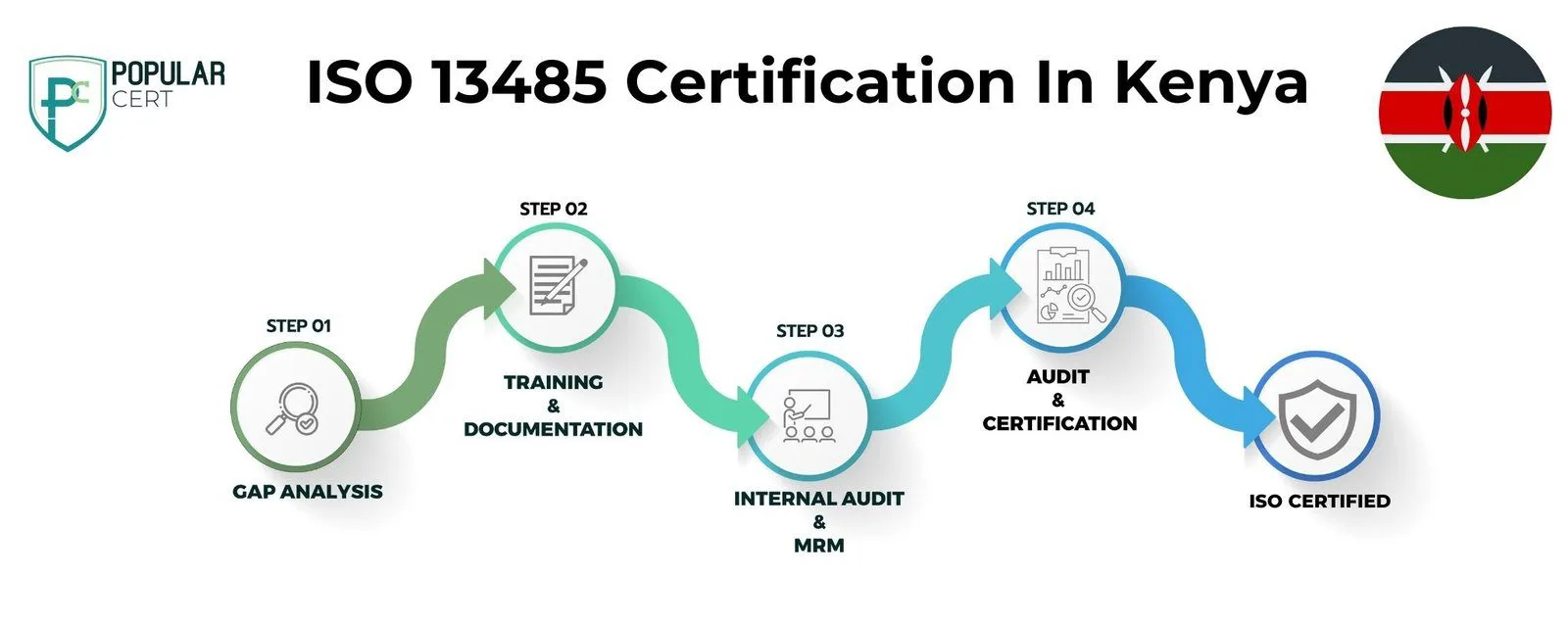
Process to Get ISO 13485 Certification In Kenya
Consultation and Gap Analysis
PopularCert’s specialists assess your organization’s specific requirements and existing systems. We conduct a thorough gap analysis to pinpoint areas needing improvement to meet ISO standards.
Planning, Documentation, and Policy Development
Following the gap analysis, we create a customized implementation plan, define resource needs, and assist in developing necessary policies and documentation. These are seamlessly integrated into your current organizational framework.
Training and Awareness
Comprehensive training ensures your team understands ISO requirements and their responsibilities in maintaining the management system effectively.
Internal Audit and Management Review
We perform internal audits to evaluate system effectiveness and address any non-conformities. A management review aligns the system with your organization’s objectives and ISO standards.
External Certification Audit and Certification
After successfully completing the external audit, your organization will earn ISO certification. This reflects your commitment to excellence, strengthens credibility, and builds lasting trust with customers and stakeholders.
Benefits Of ISO 13485 Certification In Kenya
- Improved Product Quality : ISO 13485 helps companies ensure that their medical devices meet the highest quality standards, ensuring safety and reliability for patients and healthcare providers.
- Compliance with Regulations : The certification ensures businesses comply with local and international regulations, reducing the risk of legal issues or penalties related to medical device manufacturing.
- Increased Customer Confidence : ISO 13485 certification demonstrates your commitment to quality and safety, which helps build trust with customers, regulatory bodies, and partners.
- Access to Global Markets : ISO 13485 is recognized worldwide, allowing Kenyan businesses to expand their reach and gain access to international markets for medical devices.
- Enhanced Reputation : Being ISO 13485 certified boosts your company’s reputation in the industry, signaling that you adhere to international standards and are dedicated to producing safe, high-quality products.
- Risk Management : The standard provides a structured approach to identifying and managing risks, helping businesses minimize errors and improve product safety.
- Operational Efficiency : ISO 13485 helps streamline processes, reduce waste, and enhance overall operational efficiency, leading to cost savings and improved productivity.
- Continuous Improvement : The certification encourages ongoing improvement in product development, manufacturing processes, and customer satisfaction.
Types Of ISO Certification In Kenya
- ISO Certification In Kenya
- ISO 9001 Certification
- ISO 14001 Certification
- ISO 45001 Certification
- ISO 22000 Certification
- ISO 27001 Certification
- ISO 17025 Certification
- ISO 13485 Certification
- CE Mark Certification
- ISO 20000-1 Certification
- GMP Certification
- Halal Certification
- SOC-1 Certification
- SOC-2 Certification
Get Free Consultation
Our Clients


















Which Industries Need ISO 13485 certification in Kenya
- Medical Device Manufacturers: Companies that produce surgical tools, diagnostic equipment, and hospital machines.
- Healthcare Equipment Distributors: Businesses involved in importing and supplying medical devices locally.
- Pharmaceutical Companies: Especially those producing combination products that include medical components.
- Diagnostic Labs and Testing Centers: Centers that use or develop medical testing devices.
- Dental and Optical Device Providers: Businesses offering dental instruments, lenses, and optical equipment.
Cost Of ISO 13485 Certification In Kenya
The cost of ISO 13485 certification in Kenya varies based on factors like the size of your organization, the complexity of your quality management system, and the specific medical device requirements you need to fulfill. PopularCert makes the process easier by offering customized solutions to meet your organization’s unique medical device quality needs. By partnering with PopularCert, you ensure expert guidance, a streamlined certification process, improved quality management practices, and a competitive advantage through compliance with globally recognized ISO 13485 standards in Kenya. For more information and to apply for your ISO 13485 Certification In Kenya. We will guide you through the process and provide details on the cost involved to help you get started on your ISO 13485 Certification journey with PopularCert in Kenya.
Why Choose PopularCert For ISO 13485 Certification In Kenya?
Choose PopularCert for ISO 13485 Certification in Kenya because we provide expert support that fits your business needs. Our experienced team will guide you through the certification process, making sure your medical device operations meet global quality standards. With our help, you’ll improve product safety, stay compliant with regulations, and build trust with your customers. We make the whole process simple, so your business can easily meet international standards and stand out in the medical device industry. Let PopularCert be your trusted partner on your journey to ISO 13485 Certification.
GET A FREE CONSULTATION NOW
FAQ
What is ISO 13485 Certification?
ISO 13485 is a quality management standard specifically for the medical device industry. It helps businesses ensure their products are safe, reliable, and meet regulatory requirements.
Why is ISO 13485 certification important for medical device companies in Kenya?
It helps medical device businesses in Kenya improve product quality, meet global regulatory standards, and enhance customer trust in their products.
How long does it take to get ISO 13485 certification in Kenya?
The certification process can take anywhere from a few months to a year, depending on the size of the company and the complexity of its processes.
What are the benefits of ISO 13485 certification for medical device companies?
ISO 13485 certification helps businesses ensure product quality, comply with international regulations, reduce risks, and gain a competitive edge in the medical device industry.
How do I get started with ISO 13485 certification in Kenya?
To get started, businesses need to assess their current processes, implement a quality management system, and work with a consultant to guide them through the certification process.
