ISO 13485 certification in LAGOS
Get Free Consultation
PopularCert is your trusted partner for ISO 13485 certification in Lagos, offering expert guidance to help you meet international quality standards. Achieving this certification demonstrates your dedication to providing safe, high-quality medical devices and related services.
With PopularCert, the process becomes seamless and efficient, ensuring compliance while unlocking valuable benefits for your business. Rely on our experienced consultants to deliver personalized solutions that support your success in the competitive medical device industry.
ISO 13485 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. Expand your business and ensure top quality standards with ISO 13485 Certification in Lagos. Partner with the best consultants for reliable, competitive cost.
What is ISO 13485?
ISO 13485 is an international standard that outlines the requirements for a Quality Management System (QMS) in the medical device industry. This standard is specific to medical devices and covers the entire life cycle of a device, from design and development to production, installation and servicing. It is intended to ensure that medical devices are safe and effective for their intended use. Compliance with ISO 13485 is often required for regulatory approval of medical devices in many countries around the world. The standard is designed to be flexible and scalable, allowing it to be adapted to the needs of organizations of all sizes and types within the medical device industry.
Why is ISO 13485 important in Lagos?
ISO 13485 is important for medical device manufacturers in Lagos because it provides a framework for ensuring that their products consistently meet regulatory and customer requirements. Compliance with this standard demonstrates a company’s commitment to producing safe and effective medical devices. It also helps companies meet regulatory requirements in many markets around the world and provides new business opportunities.
How to Get ISO 13485 Certification In Lagos?
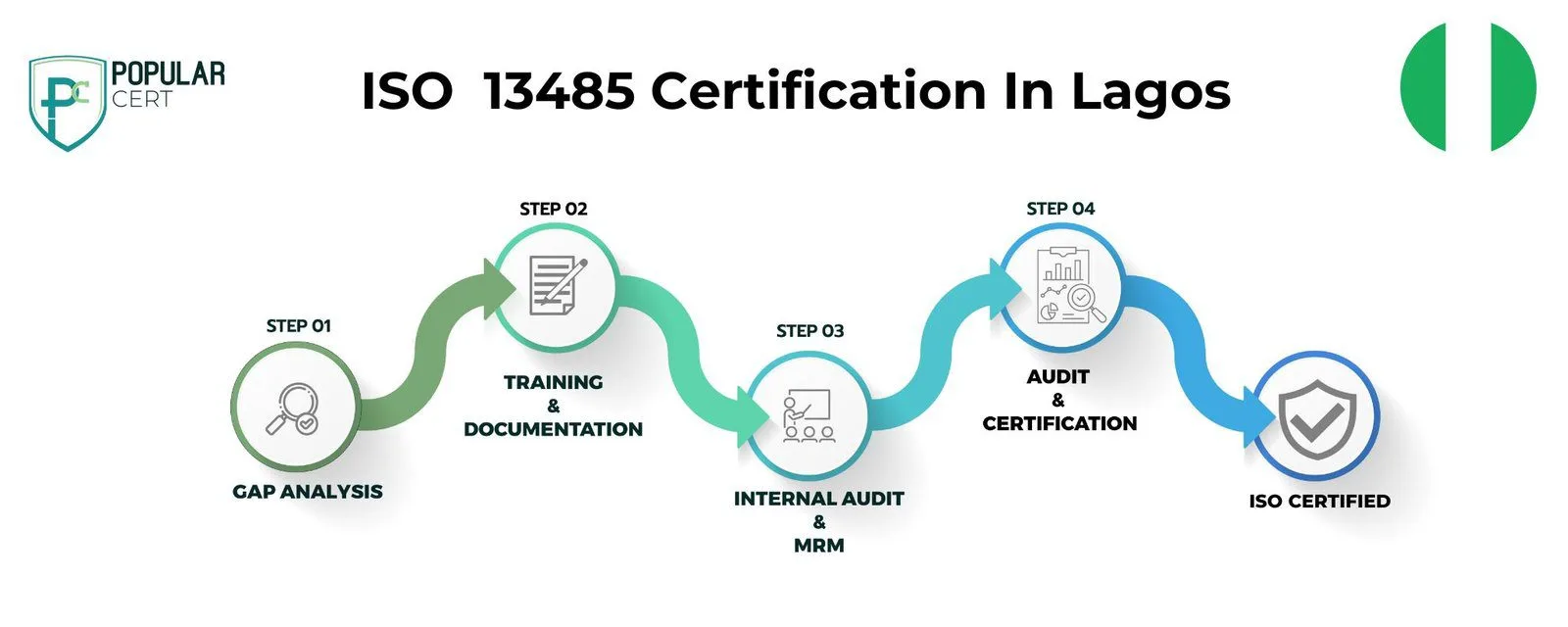
Process to Get ISO 13485 Certification In Lagos
Consultation and Gap Analysis
PopularCert’s specialists assess your organization’s specific requirements and existing systems. We conduct a thorough gap analysis to pinpoint areas needing improvement to meet ISO standards.
Planning, Documentation, and Policy Development
Following the gap analysis, we create a customized implementation plan, define resource needs, and assist in developing necessary policies and documentation. These are seamlessly integrated into your current organizational framework.
Training and Awareness
Comprehensive training ensures your team understands ISO requirements and their responsibilities in maintaining the management system effectively.
Internal Audit and Management Review
We perform internal audits to evaluate system effectiveness and address any non-conformities. A management review aligns the system with your organization’s objectives and ISO standards.
External Certification Audit and Certification
After successfully completing the external audit, your organization will earn ISO certification. This reflects your commitment to excellence, strengthens credibility, and builds lasting trust with customers and stakeholders.
Benefits Of ISO 13485 Certification In Lagos
- Enhanced product quality: ISO 13485 strongly emphasizes risk management and quality control throughout the product lifecycle. By implementing this standard, organizations establish robust processes that ensure consistent product quality, reducing the risk of defects and improving patient safety.
- Regulatory compliance : ISO 13485 aligns with global regulatory requirements for medical devices. Implementing these standards aids organizations in meeting regulatory obligations, streamlining audits and facilitating market access.
- Improved customer satisfaction : ISO 13485 certification is a mark of quality and reliability. Customers, healthcare professionals and regulatory bodies place trust in organizations that adhere to this standard, promoting confidence in the safety of their products.
- Efficient processes, documentation and streamlined operations: ISO 13485 promotes efficient processes, ensuring smooth operations throughout the organization. Organizations can eliminate redundancies, reduce errors and optimize resource allocation by standardizing these procedures.
- Improved risk management : ISO 13485 facilitates rigorous risk management in medical device manufacturing, ensuring product safety and quality throughout the supply chain. By adhering to this standard, companies mitigate risks of non-compliance, product recalls and regulatory penalties, fostering trust among customers and safeguarding public health.
- Access to the global market : ISO 13485 certification opens doors to international markets. Many countries and regulatory bodies recognize this standard as a prerequisite for market entry, enabling organizations to expand their reach and access new business opportunities.
- Continuous improvement: ISO 13485 fosters a culture of continuous improvement within organizations. By establishing metrics, conducting regular audits and implementing corrective actions, organizations can drive ongoing enhancements to their quality management system.
- Supplier relationship management: ISO 13485 emphasizes the importance of supplier evaluation and control. By implementing stringent supplier management processes, organizations can ensure the quality and reliability of external parties' raw materials, components and services.
- Competitive advantage : ISO 13485 certification provides organizations with a competitive edge in the medical device industry. It enhances brand reputation, increases market share and opens up opportunities for collaborations and partnerships, driving overall business growth.
Types Of ISO Certification In Lagos
Get Free Consultation
Our Clients


















Cost Of ISO 13485 Certification In Lagos
The cost of ISO 13485 Certification in Lagos varies based on the size of your organization, the nature of your medical device operations, and the specific scope of certification required. PopularCert makes the certification process straightforward by providing tailored solutions designed to meet your business’s unique requirements. Partnering with PopularCert ensures expert guidance, a smooth certification journey, improved operational efficiency, and a stronger market position with compliance to internationally recognized quality standards for medical devices.
Why Choose PopularCert For ISO 13485 Certification In Lagos?
Choosing PopularCert for getting yourself ISO 13485 certified in Lagos has several distinct advantages. PopularCert has a proven track record of delivering high-quality certification services, ensuring compliance with the ISO 13485 standard. Our team of experts provides personalized guidance throughout the certification process, tailored to the specific needs of your organization. When you apply for certification with PopularCert, you benefit from competitive pricing without compromising on service quality, making us a cost-effective option. Our reputation for reliability and efficiency instills confidence in clients, assuring them of a smooth and successful certification journey. For ISO 13485 Certification in Lagos, choose PopularCert, a global leader in consultancy, certification, auditing, and related services.
GET A FREE CONSULTATION NOW
FAQ
What is ISO 13485 and why is it important in Lagos?
ISO 13485 is an international standard that outlines the requirements for a quality management system specific to the medical device industry. In Lagos, adherence to ISO 13485 is crucial for ensuring the safety and efficacy of medical devices produced and distributed in the region. Compliance to this standard helps manufacturers maintain consistency, regulatory compliance and customer satisfaction, fostering trust in the industry.
What are the Benefits of ISO 13485 certification in Lagos?
ISO 13485 certification in Lagos assures compliance with international standards for quality management system in medical device manufacturing. It enhances market credibility, ensures regulatory compliance, streamlines processes, improves product quality, fosters customer trust and facilitates market access, fostering growth and competitive advantage in the medical device industry.
Who Should Get ISO 13485 Certification in Lagos?
ISO 13485 certification in Lagos is crucial for medical device manufacturers, suppliers and distributors. It ensures compliance with international quality standards, enhances product safety and fosters customer confidence. Additionally, it is essential for regulatory compliance and market access, aiding in global competitiveness.
How Does ISO 13485 Certification Work in Lagos?
ISO 13485 certification involves several steps: choosing the certification consultant and certification body, conducting an initial assessment, implementing necessary quality management system changes, undergoing audits and achieving certification upon meeting all the requirements for medical device quality management.
