ISO 13485 Certification in Dammam
Get Free Consultation
PopularCert is a trusted ISO 13485 consulting firm in Dammam, dedicated to assisting medical device companies in achieving excellence in quality management systems. ISO 13485 certification is essential for organizations in the medical device industry to ensure compliance with global regulatory standards, enhance product safety, and maintain consistent quality. By implementing ISO 13485, businesses in Dammam can build customer trust, improve operational efficiency, and expand into international markets. PopularCert offers expert guidance throughout the certification process, tailored to your organization’s needs. Apply for ISO 13485 Certification In Dammam with PopularCert and elevate your business as a leader in medical device quality.
What is ISO 13485 & why is it important in Dammam?
ISO 13485 is an internationally recognized standard for quality management systems specific to the medical device industry. It ensures organizations meet regulatory requirements and deliver safe, reliable medical devices. In Dammam, where the healthcare and medical device sectors are rapidly growing, ISO 13485 certification is vital for enhancing product quality, ensuring patient safety, and building customer trust. It helps businesses comply with global regulations, improve operational efficiency, and gain access to international markets. By adopting ISO 13485, companies in Dammam can strengthen their reputation, drive innovation, and contribute to the advancement of healthcare in the region and beyond.
How to Get ISO 13485 Certification In Dammam?
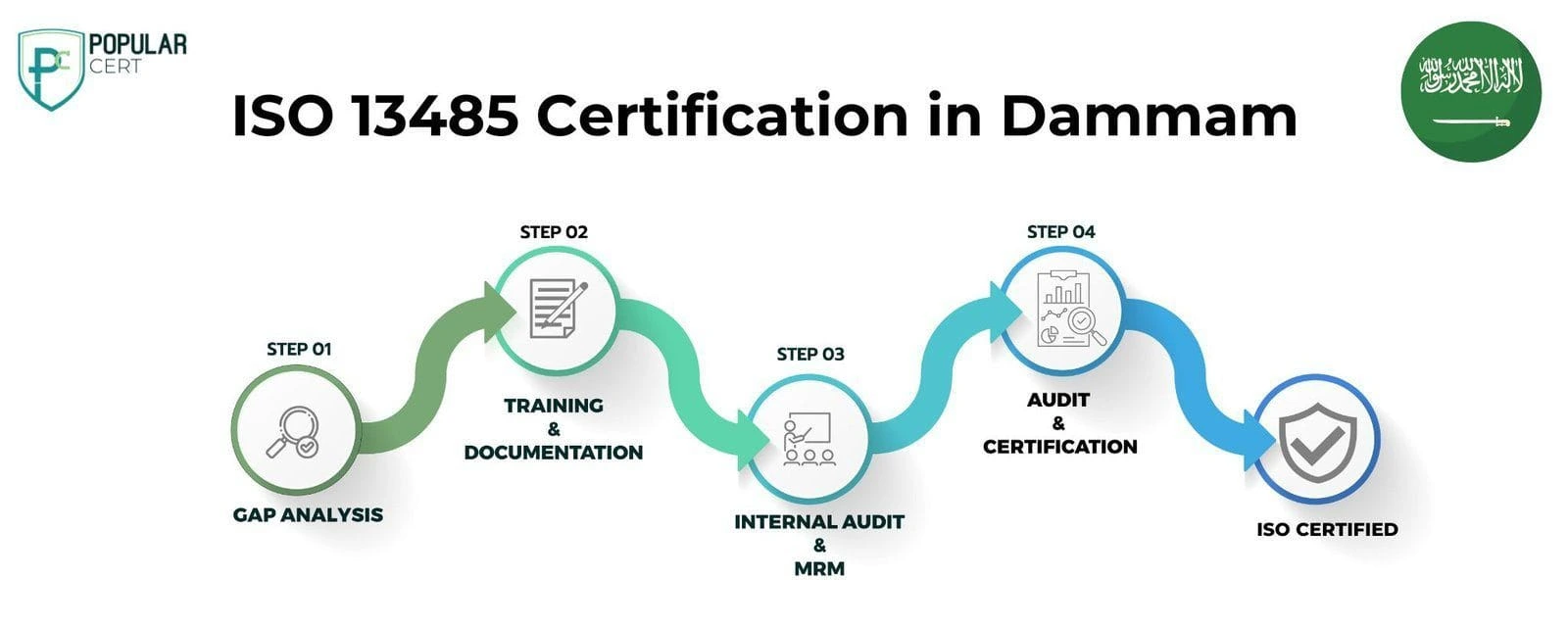
Process to Get ISO 13485 Certification In Dammam
Consultation and Gap Analysis
PopularCert’s specialists assess your organization’s specific requirements and existing systems. We conduct a thorough gap analysis to pinpoint areas needing improvement to meet ISO standards.
Planning, Documentation, and Policy Development
Following the gap analysis, we create a customized implementation plan, define resource needs, and assist in developing necessary policies and documentation. These are seamlessly integrated into your current organizational framework.
Training and Awareness
Comprehensive training ensures your team understands ISO requirements and their responsibilities in maintaining the management system effectively.
Internal Audit and Management Review
We perform internal audits to evaluate system effectiveness and address any non-conformities. A management review aligns the system with your organization’s objectives and ISO standards.
External Certification Audit and Certification
After successfully completing the external audit, your organization will earn ISO certification. This reflects your commitment to excellence, strengthens credibility, and builds lasting trust with customers and stakeholders.
Benefits Of ISO 13485 Certification In Dammam
- Compliance and regulatory assurance : It helps organizations ensure compliance with regulatory requirements in the medical device industry in Dammam. By adhering to these guidelines, the companies can demonstrate their commitment to producing safe and effective medical devices.
- Enhanced product quality : ISO 13485 helps create a culture of quality within the company, focusing on managing risks, improving processes, and always getting better. This ensures medical devices meet customer needs and regulatory standards. With improved quality, there are fewer recalls, complaints, and returns, which strengthens the company’s reputation and edge in the market.
- Improved customer satisfaction : ISO 13485 focuses on understanding and meeting customer needs. By adopting this QMS, organizations can consistently deliver what customers expect and handle feedback effectively. Happier customers lead to stronger loyalty, repeat business, and positive word-of-mouth.
- Efficiency and cost reduction : ISO 13485 encourages efficient use of resources by streamlining processes, cutting waste, and improving resource allocation. This boosts productivity, lowers costs, and increases profitability. It also promotes risk-based decision-making, helping companies focus resources on areas that impact product quality and regulatory compliance the most.
- Global market access : Certification to ISO 13485 demonstrates an organization’s commitment to quality and regulatory compliance, facilitating access to the international markets. Many regulatory authorities and customers worldwide recognize ISO 13485 certification as evidence of a robust quality management system. Obtaining this certification can help organizations expand their market reach, attract new customers and access new business opportunities globally.
- Continuous Improvement : It encourages organizations to adopt a culture of continuous improvement, where processes, products and systems are regularly reviewed and enhanced to achieve better outcomes.
Types Of ISO Certification In Dammam
Get Free Consultation
Our Clients


















How ISO 13485 Supports Dammam’s Medical Device Industry
industry that manufactures and distributes devices. There is a greater need than ever for high-quality, legally compliant medical devices because of the area’s close proximity to King Fahd Specialist Hospital and numerous healthcare investment projects.
The implementation of ISO 13485 certification in Dammam guarantees that distributors, suppliers, and manufacturers:
- Fulfill the medical device requirements set forth by the Saudi Food and Drug Authority (SFDA).
- bolster market access in the GCC and Saudi Arabia.
- Boost product dependability and patient safety.
- Become credible in the eyes of private and public healthcare purchasers.
Cost Of ISO 13485 Certification In Dammam
The cost of obtaining ISO 13485 Certification In Dammam depends on several factors, such as the size of the organization, the complexity of processes, and the scope of certification required. The cost typically includes consultancy, training, documentation, internal audits, and the final certification audit. Prices may vary based on the level of support needed throughout the process. To get a more accurate estimate based on your specific situation, contact PopularCert today.
Why Choose PopularCert For ISO 13485 Certification In Dammam?
PopularCert is a leading global consultancy with skilled experts helping businesses implement ISO standards. Our mission is to guide companies toward success by aligning their practices with global best practices. With a focus on simplicity, transparency, and quality, we ensure our clients understand every step of the process. We don’t just train teams, we enhance their implementation skills for lasting results. PopularCert has a proven track record of successful certifications. Our consultants guarantee high-quality services, and we offer competitive cost to help businesses apply for certification and secure their data effectively.
For ISO 13485 Certification In Dammam, choose PopularCert, a global leader in consultancy, certification, auditing, and related services. Once we receive your inquiry, one of our experts will respond quickly with the best solution available in the market.
Strategic Advantage:
Medical device manufacturers have a distinct competitive advantage in the local and GCC markets thanks to Dammam’s ISO 13485 certification. It increases trust with foreign buyers, expedites export compliance, and strengthens credibility with the Saudi Food and Drug Authority (SFDA). This certification is a means to long-term growth and market leadership for companies hoping to capitalize on Dammam’s expanding status as a regional medical center.
GET A FREE CONSULTATION NOW
FAQ
What is ISO 13485 and why is it important in Dammam?
ISO 13485 is an international standard that outlines the requirements for a quality management system specific to the medical device industry. In Dammam, adherence to ISO 13485 is crucial for ensuring the safety and efficacy of medical devices produced and distributed in the region. Compliance to this standard helps manufacturers maintain consistency, regulatory compliance and customer satisfaction, fostering trust in the industry.
What are the Benefits of ISO 13485 certification in Dammam?
ISO 13485 certification in Dammam assures compliance with international standards for quality management system in medical device manufacturing. It enhances market credibility, ensures regulatory compliance, streamlines processes, improves product quality, fosters customer trust and facilitates market access, fostering growth and competitive advantage in the medical device industry.
Who Should Get ISO 13485 Certification in Dammam?
ISO 13485 certification in Dammam is crucial for medical device manufacturers, suppliers and distributors. It ensures compliance with international quality standards, enhances product safety and fosters customer confidence. Additionally, it is essential for regulatory compliance and market access, aiding in global competitiveness.
How Does ISO 13485 Certification Work in Dammam?
ISO 13485 certification involves several steps: choosing the certification consultant and certification body, conducting an initial assessment, implementing necessary quality management system changes, undergoing audits and achieving certification upon meeting all the requirements for medical device quality management. To obtain ISO 13485 certification, you will need to successfully complete an Initial Certification Audit. After earning the initial certification, you will need to complete yearly surveillance audits and re-certification audits every three years to maintain your certification.
