ISO 13485 certification in dubAI
Get Free Consultation
Get ISO 13485 Certification in Dubai with PopularCert, your trusted partner for medical device compliance and quality assurance. We help manufacturers across Business Bay, Jumeirah, Al Qusais, and beyond implement ISO 13485 compliant Quality Management Systems (QMS) that meet global regulatory standards. Our services ensure product safety, consistency, and certification readiness. PopularCert also supports integration with ISO 14971 (risk management) and ISO 9001 (quality management) to boost operational excellence and accelerate access to international markets.
What Is ISO 13485 Certification?
ISO 13485 certification is an international standard for quality management systems specifically for the medical device industry. It ensures that organizations consistently meet regulatory requirements and deliver safe, effective products. This certification boosts credibility, improves product quality, and supports global market access, making it essential for manufacturers, suppliers, and service providers in the healthcare sector.
Why Is ISO 13485 Certification Important in Dubai?
- In Dubai‘s fast-growing healthcare and medical technology sectors, ISO 13485 certification is a vital mark of trust. It assures that medical device manufacturers follow strict quality and regulatory standards, which is crucial for ensuring patient safety and consistent product performance.
- With Dubai’s increasing focus on global healthcare partnerships and innovation, ISO 13485 helps companies stay competitive and export-ready. It builds confidence with regulators, clients, and end-users while reducing risks and compliance issues. For any business involved in designing, producing, or distributing medical devices, this certification is not just a regulatory need; it’s a strategic asset that aligns with Dubai’s commitment to quality healthcare solutions and international excellence.
How to get ISO 13485 Certification In Dubai?
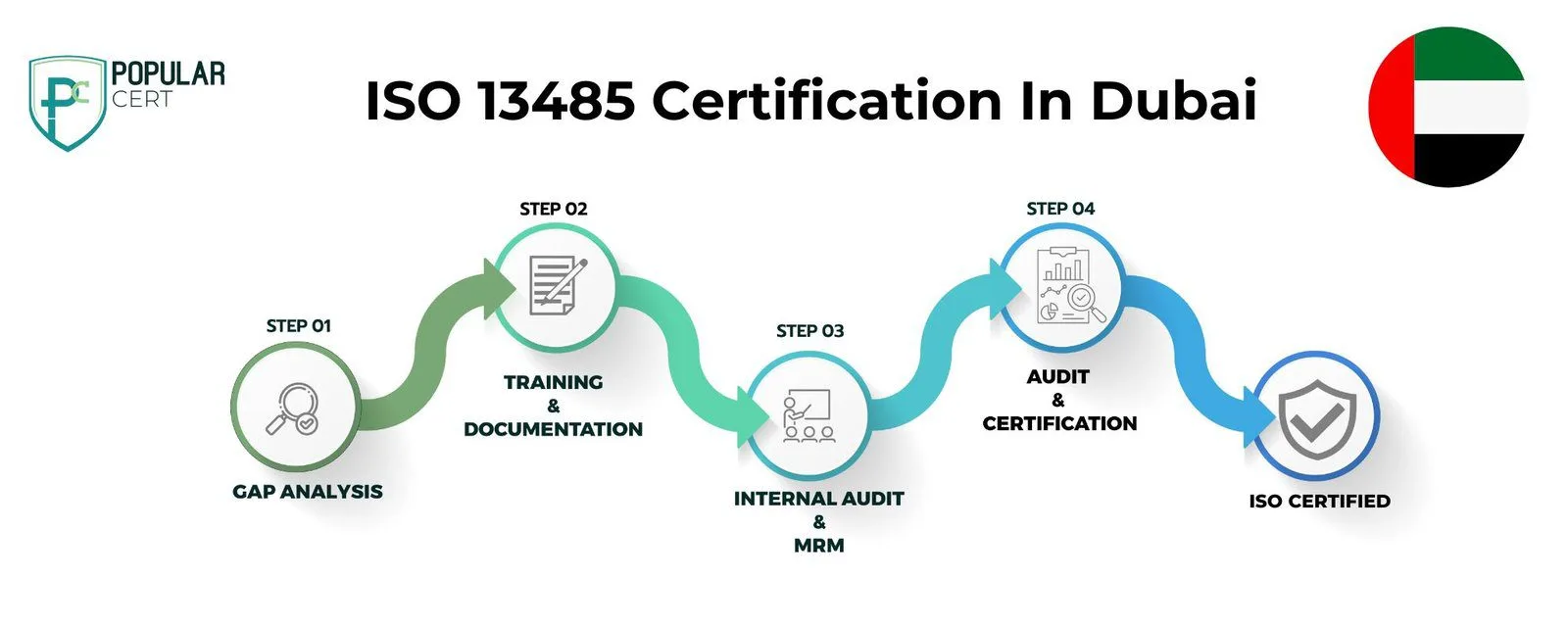
Process to Get ISO 13485 Certification In Dubai
Consultation and Gap Analysis
First and foremost, critically review the existing practices, procedures, and processes of your organization. This will assist in pointing out certain areas where your existing QMS may not completely adhere to ISO 13485. The result from such analysis would be the basis for developing a plan to fill these gaps, which will help in compliance.
Planning and Documentation Development
Establish and document an all-inclusive QMS in regard to quality manuals, SOPs, work instructions, and records. It has to spell out activities pertaining to medical device design, production, and distribution. Proper documentation will ensure consistency, traceability, and accountability throughout all the functions of an organization.
Training and Awareness
Provide relevant training packages for all employees to help acquaint them with the roles they are supposed to play in QMS and what they should know concerning ISO 13485. The training would provide not only the technical aspect but also focus on the quality management principles. Refresher courses or updates on changes to the standard shall always keep the staff knowledgeable and compliant.
Internal Audit
The QMS shall have regular internal audits to evaluate the effectiveness of the QMS and ensure that it complies with the requirements as enunciated in ISO 13485. Such audits should be planned, systematic, and documented. They need to cover all the areas of QMS, and analysis of the findings is necessary for improvement action. Internal audits prepare an organization in advance for external audits by pointing out and rectifying problems.
Certification Audit
Secure a formal certification audit from an independent accredited certification body. The auditors will judge the effectiveness of your QMS against the normative requirements for ISO 13485. This is the cardinal stage in attaining certification, outlining a review of your documentation, processes, and implementation practices.
Benefits of ISO 13485 Certification in Dubai
- Regulatory Compliance: Ensures adherence to local and international regulatory requirements, facilitating market entry and legal compliance.
- Improved Product Quality: Promotes consistent production of safe and high-quality medical devices, enhancing customer confidence.
- Global Market Access: Enables companies to expand into international markets where ISO 13485 is a recognized standard.
- Risk Management: Reduces product-related risks through robust quality management practices and controlled processes.
- Competitive Advantage: Demonstrates commitment to excellence, boosting reputation and providing a market edge.
- Operational Efficiency: Enhances internal processes, reduces waste, and promotes a culture of continual improvement.
Types Of ISO Certification In Dubai
Get Free Consultation
Our Clients


















Cost of ISO 13485 Certification in Dubai
The cost of ISO 13485 certification in Dubai varies depending on the size of your medical device company, operational complexity, and current compliance level. Many businesses start with a free ISO consultation in Dubai to understand their specific needs. If you’re searching for ISO 13485 consultants near me in Dubai, here are the key pricing factors:
- Company size and number of employees
- Scope of medical device design and production
- Existing quality management systems
- Required documentation and training
- Internal audit and process improvements
- Certification body and audit duration
- Number of sites involved
Partnering with the best ISO certification company in Dubai ensures efficient implementation, cost-effective services, and smooth certification aligned with international standards.
Why choose PopularCert for ISO 13485 Certification in Dubai?
PopularCert is a trusted choice for ISO 13485 certification in Dubai, offering tailored solutions for medical device companies aiming to meet international regulatory standards. We simplify the ISO certification process in Dubai, helping businesses ensure product safety, quality, and regulatory compliance. Our consultants provide industry-specific guidance to align your quality management system with ISO 13485 requirements.
- Expert ISO 13485 consultants in Dubai
- Full support from gap analysis to certification
- Specialized in medical devices and healthcare sector
- Risk-based approach to quality assurance
- Reliable ISO certification services Dubai
Choose PopularCert to strengthen your compliance and quality standards in Dubai’s medical sector.
GET A FREE CONSULTATION NOW
FAQ
What is ISO 13485 certification in Dubai and why is it important for medical businesses?
ISO 13485 certification in Dubai ensures that medical device companies follow strict international standards for quality and safety. It’s important for businesses that want to build trust, meet UAE regulatory requirements, and access global markets.
Who should get ISO 13485 certified in Dubai?
Manufacturers, distributors, suppliers, and service providers in the medical device industry in Dubai should consider ISO 13485 certification. It helps demonstrate commitment to quality control, risk management, and regulatory compliance.
How much does ISO 13485 certification cost in Dubai?
The cost of ISO 13485 certification in Dubai depends on your organization’s size, complexity, and readiness. At PopularCert, we provide cost-effective, tailored solutions to help you achieve certification smoothly and efficiently.
How long does it take to obtain ISO 13485 Certification?
Getting ISO 13485 Certification isn’t always quick. It involves various factors. Your group’s size, complexity, and preparedness play a big role. Usually, it could be a couple of months before all is done successfully.
