ISO 13485 Certification in Nairobi
Get Free Consultation
ISO 13485 certification is essential for medical device companies in Nairobi. It establishes a quality management system (QMS) that ensures product safety and regulatory compliance.
PopularCert is a global leader in consulting, dedicated to helping businesses achieve international standards with simplicity, honesty, and clarity. Our expert team ensures clients in Kenya understand and implement ISO requirements effectively. To achieve certification, organizations must implement quality processes throughout the entire medical device lifecycle, from design to post-market activities. An accredited certification body conducts a thorough assessment to verify compliance with the ISO 13485 standard.
Simplify your lab’s testing and calibration with ISO 13485 Certification in Nairobi. Enhance medical device standards with expert consultants at the best cost. Obtaining this certification enhances a company’s reputation, builds customer trust, and opens up new business opportunities in the growing medical device market in Kenya.
Importance of ISO 13485 Certification for companies in Nairobi
ISO 13485 certification is essential for medical device companies in Nairobi as it ensures compliance with international quality and safety standards. This certification not only enhances regulatory compliance but also improves market access by demonstrating a commitment to delivering high-quality, reliable products. It streamlines operational processes, reduces risks, and boosts customer confidence in the safety and effectiveness of medical devices. Furthermore, ISO 13485 certification strengthens a company’s reputation, fosters trust among stakeholders, and supports public health goals by ensuring that products meet stringent quality requirements. Achieving this certification positions companies as industry leaders while contributing to better healthcare outcomes in Nairobi.
How to Get ISO 13485 Certification in Nairobi ?
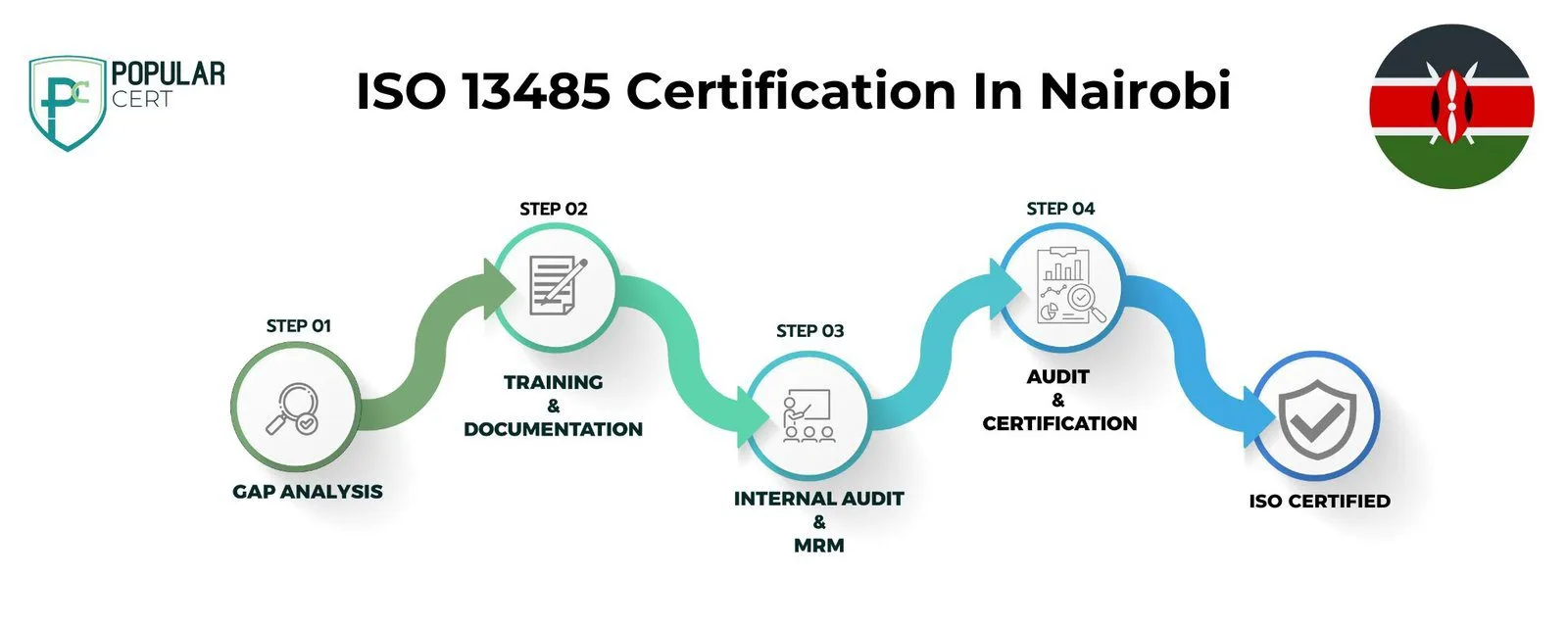
Process to Get ISO 13485 Certification in Nairobi
Consultation and Gap Analysis
PopularCert’s experts begin by assessing your organization's specific needs and current systems. We conduct a comprehensive gap analysis to identify areas that require improvement in order to comply with ISO 13485 standards.
Planning, Documentation, and Policy Development
After the gap analysis, we develop a tailored implementation plan that outlines the necessary steps and resources. We assist in creating policies and documentation that integrate smoothly into your existing organizational framework, ensuring compliance with ISO 13485.
Training and Awareness
We provide thorough training to ensure your team fully understands ISO 13485 requirements and their roles in effectively maintaining the management system.
Internal Audit and Management Review
Internal audits are conducted to assess system performance and identify any non-conformities. A management review ensures the system aligns with your organization’s goals and ISO 13485 standards.
External Certification Audit and Certification
Once the internal process is complete, we support your organization in undergoing the external audit by an accredited certification body. Upon successful audit completion, your organization will be awarded ISO 13485 certification, demonstrating your commitment to quality management in medical devices and enhancing your credibility with customers and stakeholders.
Benefits of ISO 13485 Certification in Nairobi
- Enhanced Quality Management: ISO 13485 certification helps organizations improve the quality and reliability of their medical devices and related services. It ensures your products meet regulatory requirements and customer expectations, ultimately leading to better quality control throughout the production process.
- Regulatory Compliance: Achieving ISO 13485 certification demonstrates that your organization adheres to international regulations and standards for medical devices. This helps in meeting legal and regulatory requirements in Nairobi, and across international markets, making it easier to do business globally.
- Increased Market Access: ISO 13485 certification enhances your reputation and gives you a competitive edge. Many customers and regulatory bodies prefer to do business with certified organizations, opening doors to new markets, both locally in Nairobi and internationally.
- Risk Management: ISO 13485 places a strong emphasis on risk management, helping you identify, assess, and mitigate potential risks related to medical devices. This proactive approach reduces the likelihood of defects, recalls, or safety issues.
- Customer Confidence: By demonstrating your commitment to quality management, ISO 13485 certification enhances customer trust and confidence in your products. This can result in increased customer satisfaction, loyalty, and retention.
Types Of ISO Certification In Nairobi
Get Free Consultation
Our Clients


















Cost to get ISO 13485 Certification in Nairobi
The cost of ISO 13485 certification in Nairobi varies depending on your organization’s size and complexity. Common expenses include consultation fees, training, documentation preparation, and audit charges. For a customized quote, reach out to PopularCert.
Simplify your lab’s testing and calibration with ISO 13485 Certification in Nairobi. Enhance medical device standards with expert consultants at the best cost. Learn how to get ISO 13485 certification and apply it with expert support from trusted consultants.
Why Choose PopularCert For ISO 13485 Certification in Nairobi?
- Quick Certification: Streamlined processes for efficient certification.
- Customized Solutions: Tailored services to meet specific ISO 13485 requirements.
- Continuous Support: Ongoing assistance to maintain compliance and standards.
By choosing PopularCert, businesses not only achieve ISO 13485 certification but also benefit from the numerous advantages of ISO standards, paving the way for long-term success.
GET A FREE CONSULTATION NOW
FAQ
What is ISO 13485 Certification?
ISO 13485 is a globally recognized standard for quality management systems (QMS) specific to the design, production, and maintenance of medical devices. Achieving ISO 13485 certification ensures that an organization is compliant with regulatory requirements and is committed to consistent quality.
Why is ISO 13485 Certification important for medical device manufacturers?
ISO 13485 certification is crucial for medical device manufacturers as it ensures compliance with regulatory requirements and enhances product safety. It improves processes, reduces risks, and builds trust with customers and regulatory bodies.
How can my organization apply for ISO 13485 Certification in Nairobi?
To apply for ISO 13485 certification, contact a certified consultant who will guide you through the process, including gap analysis, documentation, internal audits, and management reviews, ensuring your company is ready for the certification audit.
What are the benefits of ISO 13485 Certification for my business?
ISO 13485 certification offers numerous benefits, such as improved process efficiency, better compliance with regulatory standards, enhanced product quality, reduced costs, and increased customer satisfaction. It can also open doors to new markets and improve business reputation.
What is the process for ISO 13485 Certification?
The process for ISO 13485 certification involves a gap analysis, followed by creating and implementing quality management system documentation, conducting staff training, performing internal audits, and completing the certification audit with an accredited body. Once successful, your organization will receive the ISO 13485 certificate.
