ISO 17025 Certification in Nairobi
Get Free Consultation
ISO 17025 Certification in Nairobi helps laboratories demonstrate their technical competence and the ability to produce precise and accurate test and calibration results. As the international standard for testing and calibration laboratories, ISO 17025 ensures that labs in Nairobi meet global benchmarks for quality and reliability. Achieving ISO 17025 certification boosts confidence among clients, regulators, and stakeholders, while improving internal processes and data integrity. This certification not only enhances credibility but also opens doors to national and international partnerships, ensuring sustained growth and trust in the laboratory’s services.
What Is ISO 17025?
ISO 17025 certification is an international standard specifically designed for testing and calibration laboratories. It proves that a lab is technically competent and can produce accurate and reliable test results.
This certification focuses on both the quality management system and the technical requirements of a laboratory, including equipment calibration, staff skills, testing methods, and result validity.
In simple terms, ISO 17025 ensures that a lab can consistently deliver trustworthy and precise results, which is essential for industries like healthcare, manufacturing, food safety, environment, and research. It’s often required for labs working with government, regulatory bodies, or global clients.
Why is ISO 17025 Essential for lab testing?
It plays a key role in tech and trades. Both ISO and IEC 17025 help set standard methods and smooths cooperation between labs and other groups. It also supports results being accepted globally. Benefits span from business strategy to internal growth. A few standout points include boosting client trust.
Labs accredited to ISO or IAC 17025-2017 show they can give consistent, valid results. This proves the team’s knowledge and all results can be linked back to the global system of units. Being certified is handy it proves lab competency and reliability it helps with promotions and staffing while improving customer satisfaction. Plus, it helps labs work more efficiently and effectively.
How to Get ISO 17025 Certification in Nairobi ?
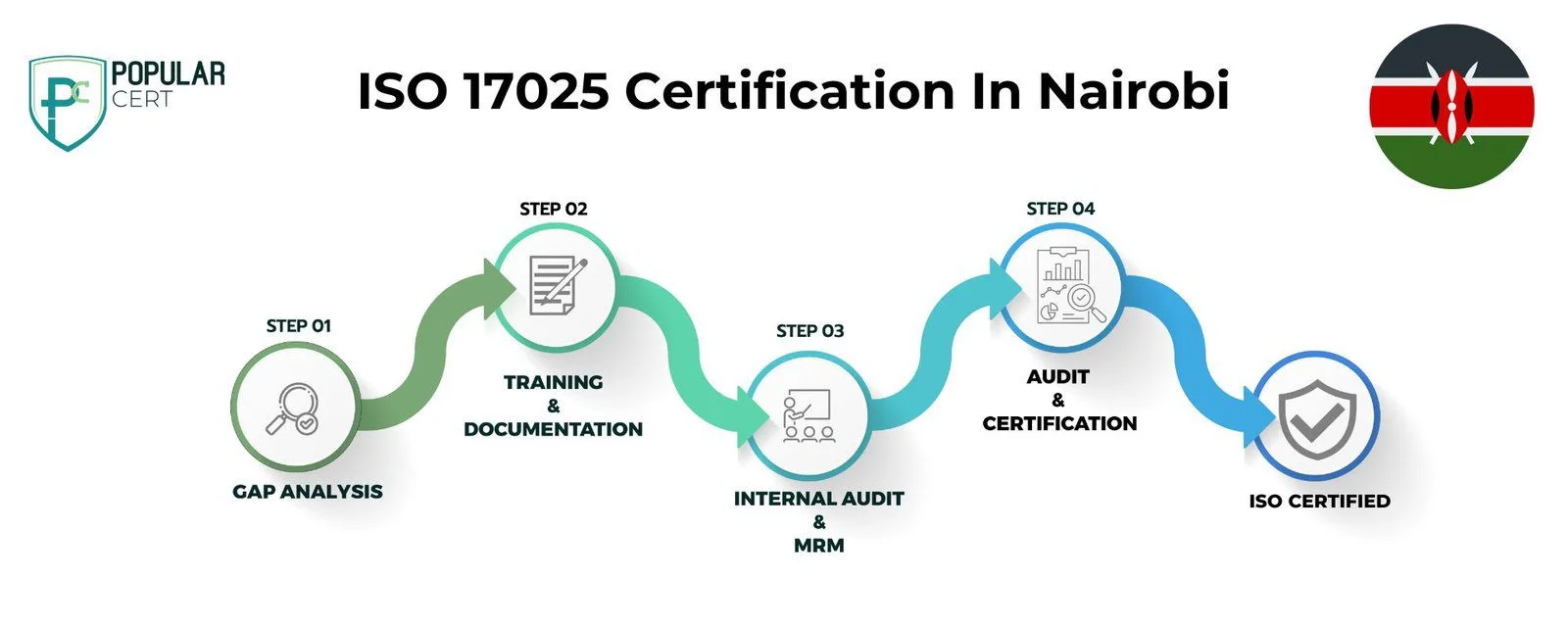
Process to Get ISO 17025 Certification in Nairobi
Consultation and Gap Analysis
PopularCert’s experts evaluate your organization’s unique needs and current systems. We conduct a detailed gap analysis to identify areas requiring improvement to align with ISO 17025 standards.
Planning, Documentation, and Policy Development
Based on the gap analysis, we design a tailored implementation plan, define resource requirements, and support the creation of essential policies and documentation. These are seamlessly incorporated into your existing laboratory management framework.
Training and Awareness
We provide thorough training to ensure your team comprehends ISO 17025 requirements and their roles in maintaining an effective laboratory management system.
Internal Audit and Management Review
Our team conducts internal audits to assess the system’s performance and resolve non-conformities. A management review ensures the laboratory management system aligns with your organization’s goals and ISO 17025 requirements.
External Certification Audit and Certification
Upon successful completion of an external audit by an accredited certification body, your laboratory will achieve ISO 17025 certification. This demonstrates your commitment to competency, accuracy, and compliance with international standards, enhancing your credibility and trustworthiness in the field.
Benefits of ISO 17025 Certification in Nairobi
- Enhanced Laboratory Competence: ISO 17025 ensures your laboratory meets global standards, demonstrating technical competence and accuracy in test and calibration results.
- Increased Credibility: Certification builds trust with clients and stakeholders by proving your laboratory operates to internationally recognized standards.
- Improved Operational Efficiency: Implementing ISO 17025 streamlines laboratory processes, reducing errors, minimizing waste, and enhancing overall efficiency.
- Compliance with Regulations: Certification helps laboratories meet regulatory and statutory requirements, avoiding penalties and ensuring smooth operations.
- Global Recognition: ISO 17025 certification ensures your laboratory’s results are recognized and accepted worldwide, opening doors to new markets and collaborations.
- Customer Satisfaction: By delivering reliable and accurate results, certified laboratories improve client confidence, leading to stronger relationships and repeat business.
- Competitive Edge: Achieving ISO 17025 certification distinguishes your laboratory from competitors, attracting more clients and projects.
- Risk Management: The certification process helps identify and mitigate risks in testing and calibration processes, ensuring consistent quality and safety.
Types Of ISO Certification In Nairobi
Get Free Consultation
Our Clients


















Which Industries Need ISO 17025 Certification In Nairobi?
- Testing Laboratories: Labs performing chemical, biological, or mechanical testing for any industry need ISO 17025 to prove their accuracy and reliability.
- Calibration Laboratories: Labs that calibrate measuring instruments and equipment (like pressure gauges, thermometers, or scales) use ISO 17025 to ensure precision and traceability.
- Pharmaceutical & Biotechnology: These industries require strict lab testing of drugs and materials, making ISO 17025 essential for proving lab competency and regulatory compliance.
- Food and Beverage Industry: Labs that test food for safety, quality, and nutritional content need ISO 17025 to ensure consistent and credible results, especially for export compliance.
- Environmental Testing: Environmental labs analyzing air, water, or soil samples for pollution and safety follow ISO 17025 to maintain data credibility for regulatory bodies.
- Automotive and Aerospace: These industries use ISO 17025-accredited labs for material testing, product validation, and calibration to meet safety and quality standards.
- Electrical and Electronics: Labs testing electrical safety, EMC compliance, or product performance require ISO 17025 to meet international and national certification requirements.
Cost to obtaining ISO 17025 certification in Nairobi
The cost of ISO 17025 certification in Nairobi varies based on factors such as your laboratory’s size, scope, complexity, and the selected accreditation body. PopularCert offers comprehensive services, including consultations, documentation preparation, staff training, implementation support, internal audits, and certification fees, ensuring a seamless certification process. Our solutions are customized to meet your laboratory’s specific requirements, providing efficient and cost-effective outcomes. With a commitment to quality, PopularCert delivers top-notch ISO 17025 certification services in Nairobi at an affordable cost.
Simplify your lab’s testing and calibration process with ISO 17025 Certification in Nairobi. Learn how to get certified and how to apply through expert consultants who offer quality services at the best cost.
Why choose PopularCert for ISO 17025 Certification in Nairobi?
PopularCert simplifies the ISO 17025 certification process for laboratories in Nairobi, providing a smooth and hassle-free experience. We begin with a detailed consultation to understand your laboratory’s goals, challenges, and requirements. Based on this, we create a tailored plan with clear steps and timelines to guide you through the process. Our team offers ongoing support to ensure seamless implementation, with transparent and affordable pricing that includes no hidden costs. With a focus on timely delivery, we ensure your certification is completed efficiently within the agreed timeframe. Achieve ISO 17025 certification with ease—contact PopularCert today!
GET A FREE CONSULTATION NOW
FAQ
What is ISO 17025 Certification?
ISO 17025 is the international standard that specifies requirements for the competence of testing and calibration laboratories, ensuring accurate and reliable results.
Why is ISO 17025 Certification important for laboratories in Nairobi?
ISO 17025 Certification enhances your laboratory’s credibility, ensures compliance with global standards, improves accuracy, and increases customer confidence in your testing and calibration services.
How can I get ISO 17025 Certification in Nairobi?
To get ISO 17025 Certification, follow these steps:
- Conduct a gap analysis to assess current processes.
- Develop and implement a quality management system.
- Train staff on ISO 17025 requirements.
- Perform internal audits.
- Undergo an external audit by an accredited certification body.
How do I apply for ISO 17025 Certification?
To apply, contact a certification consultant like PopularCert. They will guide you through the documentation, process implementation, and application submission to an accredited certification body.
