ISO 13485 certification in PORT HARCOURT
Get Free Consultation
PopularCert is your reliable partner for ISO 13485 certification in Port Harcourt, providing expert guidance and support to medical device manufacturers and related industries. We specialize in ISO 13485 (Quality Management for Medical Devices) and offer affordable, practical solutions tailored to meet the needs of businesses in manufacturing, healthcare, and more. Our experts ensure your organization achieves compliance with international standards and enhances operational efficiency.
ISO 13485 is the internationally recognized standard for Quality Management Systems (QMS) specifically designed for organizations involved in the medical devices industry. It provides a structured framework to ensure the consistent production of safe, high-quality, and reliable medical devices. This certification demonstrates a company’s commitment to meeting regulatory requirements, reducing risks, and ensuring patient safety.
Why ISO 13485 Certification is important for you and your business in Port Harcourt.
ISO 13485 certification is a necessity for businesses that want to operate on a global scale in the medical device industry. This certification ensures that your organization adheres to strict regulatory and quality standards, which is vital for gaining trust from customers, regulators, and other stakeholders. It also helps to demonstrate that your products are safe, reliable, and meet international market requirements.
In addition, certification supports business growth by making it easier to enter new markets, maintain compliance with regulatory laws, and avoid penalties for non-compliance. It showcases your dedication to delivering quality, safe products that protect patients and end-users.
How to Get ISO 13485 Certification in Port Harcourt?
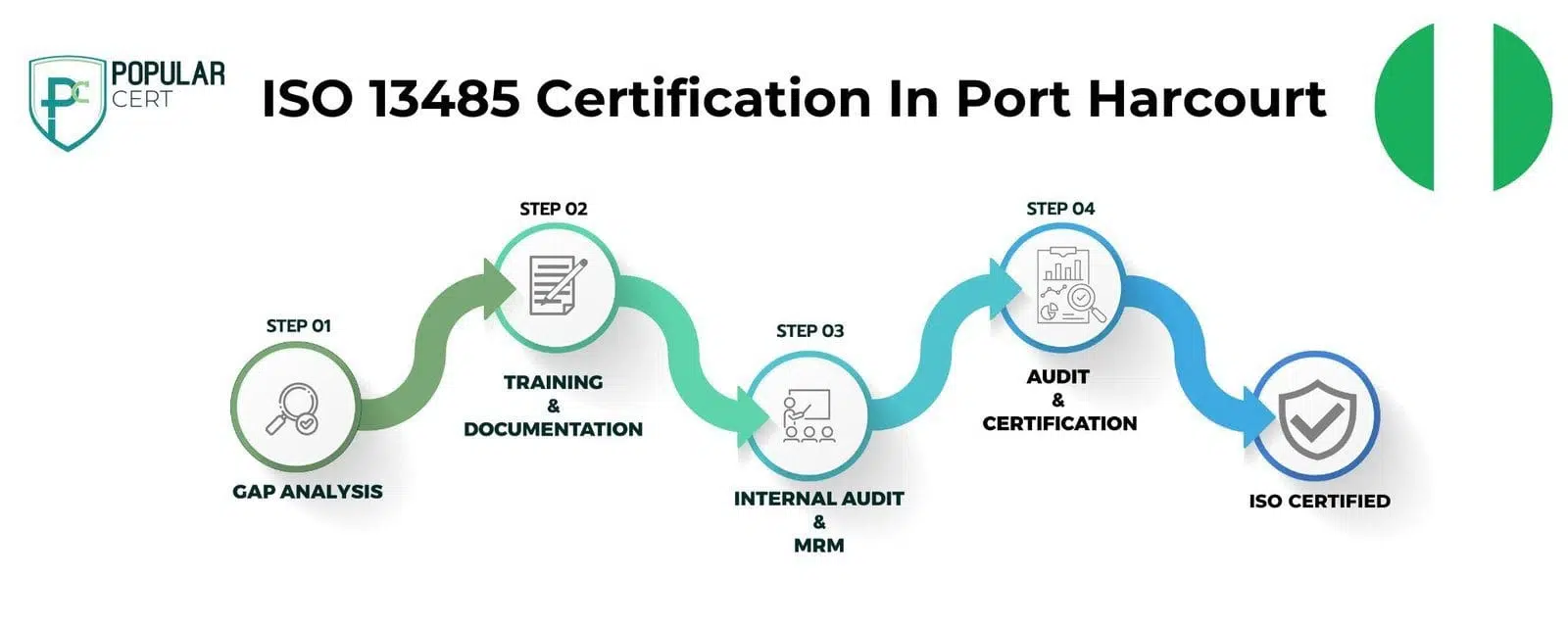
Process to Get ISO 13485 Certification In Port Harcourt
Consultation and Gap Analysis
PopularCert’s experts begin by understanding your organization’s needs and current management practices. We then conduct a gap analysis to identify areas requiring improvement for ISO 13485 certification in Port Harcourt. This ensures your organization is well-prepared to meet international standards in quality management for medical devices, ensuring compliance and readiness for certification.
Planning, Documentation, and Policy Development
Based on the gap analysis, we develop a detailed implementation plan for ISO 13485 certification in Port Harcourt. We allocate necessary resources and assist in creating the essential policies and documentation required. These policies and procedures are seamlessly integrated into your existing framework, ensuring compliance with medical device quality management standards and effective implementation.
Training and Awareness
We provide comprehensive training for your staff, ensuring they understand the requirements of ISO 13485 certification and their role in effectively implementing and maintaining the quality management system for medical devices. Our training programs are tailored to meet the specific needs of organizations in Port Harcourt, empowering your team to successfully achieve and sustain ISO 13485 certification.
Internal Audit and Management Review
After implementing the ISO 13485 management system, we conduct an internal audit to assess its efficiency and identify any non-conformities. Following this, a management review is carried out to ensure the system aligns with your organization's goals and compliance requirements for medical devices in Port Harcourt, ensuring readiness for certification.
External Certification Audit and Certification
After successfully completing the external audit by the certification body, your organization will be awarded ISO 13485 certification. This certification highlights your commitment to high standards in medical device management and continuous improvement. It demonstrates your dedication to excellence, enhances your credibility, and builds customer trust, especially for organizations in Port Harcourt.
Benefits of ISO 13485 Certification in Port Harcourt
- High-Quality Medical Devices : Certification ensures your products are consistently designed and manufactured to meet stringent safety and quality standards. This minimizes defects, reduces product recalls, and builds confidence in the reliability of your medical devices.
- Regulatory Compliance : Align your business with local, regional, and international regulatory requirements, such as EU MDR (Medical Device Regulation) and FDA standards. This not only avoids costly fines and penalties but also ensures your products are market-ready and legally compliant.
- Market Expansion Opportunities : ISO 13485 certification is globally recognized, enabling businesses to enter new markets with ease. It enhances credibility with customers, distributors, and regulators, helping you grow your business beyond local boundaries.
- Improved Customer Trust : Certification proves your dedication to delivering safe and effective medical devices. This commitment builds long-term trust with customers, suppliers, and other stakeholders, strengthening your reputation in the industry.
- Operational Efficiency : By implementing ISO 13485, businesses can streamline their internal processes, reduce production errors, and eliminate waste. This leads to improved productivity, lower costs, and a more efficient operation overall.
- Risk Mitigation : The standard emphasizes identifying and managing risks throughout the product lifecycle. This proactive approach helps prevent potential issues, protects patient safety, and ensures compliance with regulatory expectations.
- Competitive Advantage : ISO 13485 certification sets your business apart by demonstrating adherence to the highest industry standards. It gives you an edge over competitors, positioning your company as a trusted provider of high-quality medical devices.
Types Of ISO Certification In Port Harcourt
Get Free Consultation
Our Clients


















ISO 13485 Certification Relevance by Industry in Port Harcourt
Port Harcourt is emerging as a focal point for healthcare and biomedical development within Nigeria, and demands for standard practices within the medical device and healthcare sectors are increasing. ISO 13485 certification in Port Harcourt is especially important for companies that are operating in regulated environments and looking to meet quality expectations at both domestic and international levels. Many industries in the Port Harcourt area can see significant gains from implementing a Quality Management System that suits their operations:
- Medical Device manufacturers: ISO 13485 certification is needed to manufacture controlled products, from syringes to diagnostic devices, while satisfying the safety of the product, providing consistency of the products made, and meeting regulatory requirements from NAFDAC, SON or other relevant regulatory departments.
- Diagnostic Laboratories: Laboratories involved with the development or use of in-vitro diagnostic devices can provide additional accuracy and credibility to their work, with ISO 13485 standards.
- Pharmaceutical Packaging Companies: Companies accountable for providing a sterile product, and tamper-proof packaging for medical devices or products are essential in ensuring confidence and acceptance into international markets.
- Surgeon Tool Suppliers: ISO 13485 certification ensures products are handled correctly, stored in safe and secured conditions, and products are not being compromised during distribution of surgical tools, which reduces risk to the client or institution during use.
- Biomedical Engineering Services: ISO 13485 certification provides assurance to maintain good records of documentation, calibration, and servicing of medical equipment, making it essential for long-term contract commitments for clients and hospital collaboration pursuits within Port Harcourt.
For companies operating in these areas, ISO 13485 certification in Port Harcourt isn’t just a regulatory requirement it’s a strategic vehicle for the growth of the business, risk avoidance, and local and global market expansion.
Cost of ISO 13485 Certification in Port Harcourt
The cost of ISO 13485 certification in Port Harcourt depends on the size of your organization, the complexity of your management system, and its current level of compliance. Typical expenses include gap analysis, training, documentation preparation, audits, and implementation support. PopularCert offers tailored and cost-effective solutions to help businesses in Port Harcourt achieve ISO 13485 certification, ensuring compliance with international standards and enhancing operational efficiency.
Why Choose PopularCert For ISO 13485 Certification in Port Harcourt?
PopularCert is a globally recognized consulting company specializing in ISO 13485 certification, advisory, and auditing services. We are the trusted choice for organizations seeking ISO 13485 certification due to our experienced, ethical consultants and proven success record. For ISO 13485 certification in Port Harcourt, choose PopularCert, a leader in consultancy, certification, and auditing services.
Conclusion
ISO 13485 certification in Port Harcourt is an important asset for any company in the medical and healthcare industries looking for global quality and regulatory compliance. It helps the organization become more efficient, it creates “safe” products, it creates trust, and more. PopularCert has tremendous local knowledge and experience, combined with verified global experience that can be your best resource for the certification journey!
GET A FREE CONSULTATION NOW
FAQ
What is ISO 13485 and why is it important in Port Harcourt?
What are the Benefits of ISO 13485 certification in Port Harcourt?
Who Should Get ISO 13485 Certification in Port Harcourt?
How Does ISO 13485 Certification Work in Port Harcourt?
ISO 13485 certification involves several steps: choosing the certification consultant and certification body, conducting an initial assessment, implementing necessary quality management system changes, undergoing audits and achieving certification upon meeting all the requirements for medical device quality management.
