ISO 13485 certification in Muscat
Get Free Consultation
PopularCert is committed to guiding organizations through the ISO 13485 certification process, ensuring seamless compliance and market readiness. ISO 13485 certification in Muscat is essential for organizations in the medical device sector, ensuring that the design, production, installation, and delivery of medical equipment meet rigorous international quality standards. Demonstrating a dedication to quality and compliance through ISO 13485 certification can bolster your reputation and build customer trust, as it is often a prerequisite for operating in numerous countries. This certification opens doors to new markets and business opportunities.
Why ISO 13485 is Important in Muscat?
ISO 13485 is vital in Muscat as it ensures that medical device manufacturers meet strict international quality standards, which is crucial for regulatory compliance. This certification enhances the reputation of organizations, building trust with consumers, healthcare professionals, and regulatory bodies. Achieving ISO 13485 provides access to global markets and opportunities, opening doors for international partnerships and business expansion. It also leads to improvements in quality management systems, promoting greater operational efficiency and product reliability. With ISO 13485, Muscat can strengthen its healthcare sector, ensuring safer and more effective medical products for its population.
How to Get ISO 13485 Certification In Muscat?
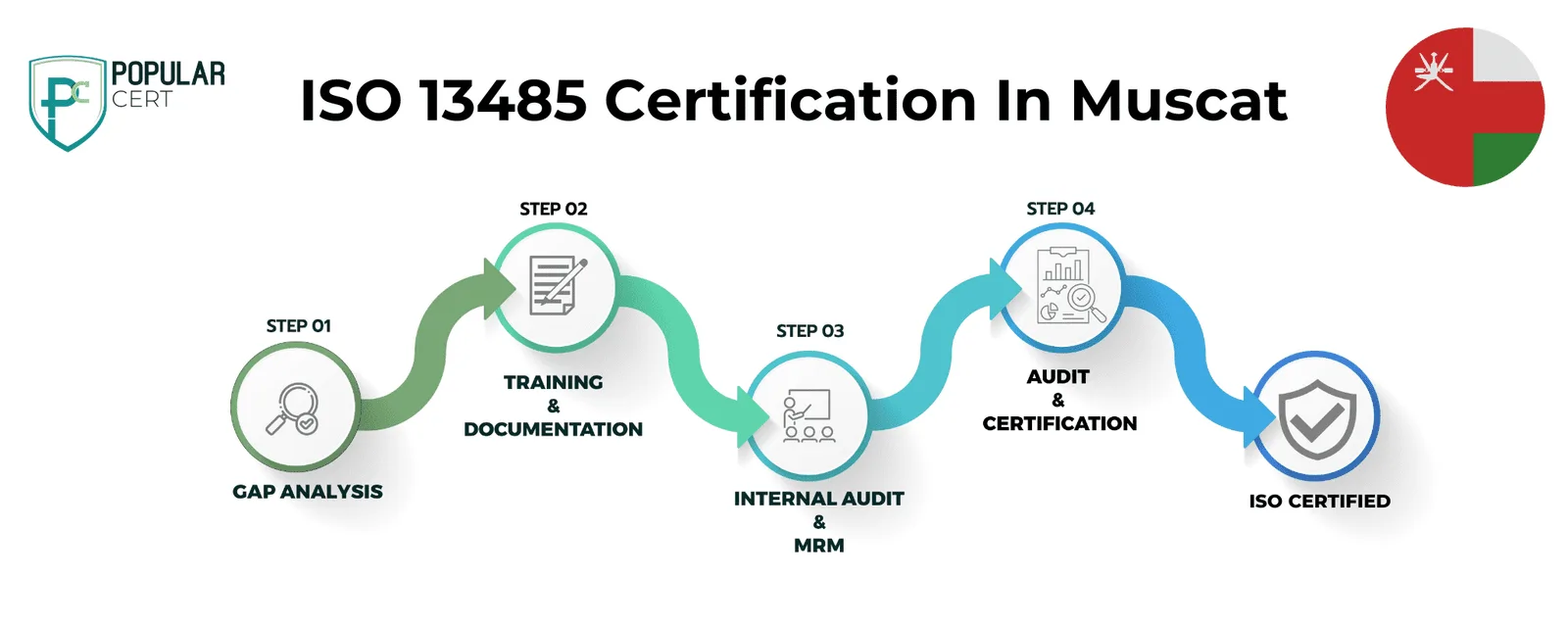
Process to Get ISO 13485 Certification In Muscat
Consultation and Gap Analysis
PopularCert’s specialists assess your organization’s specific requirements and existing systems. We conduct a thorough gap analysis to pinpoint areas needing improvement to meet ISO standards.
Planning, Documentation, and Policy Development
Following the gap analysis, we create a customized implementation plan, define resource needs, and assist in developing necessary policies and documentation. These are seamlessly integrated into your current organizational framework.
Training and Awareness
Comprehensive training ensures your team understands ISO requirements and their responsibilities in maintaining the management system effectively.
Internal Audit and Management Review
We perform internal audits to evaluate system effectiveness and address any non-conformities. A management review aligns the system with your organization’s objectives and ISO standards.
External Certification Audit and Certification
After successfully completing the external audit, your organization will earn ISO certification. This reflects your commitment to excellence, strengthens credibility, and builds lasting trust with customers and stakeholders.
Benefits Of ISO 13485 Certification In Muscat
Types Of ISO Certification In Muscat
Get Free Consultation
Our Clients


















- Regulatory assurance and compliance : It assists organisations in making sure that the regulations governing the Muscat medical device business are followed. The companies can show that they are dedicated to making safe and efficient medical equipment by following these principles.
- Improved product quality : It encourages a qualityconscious culture across the whole company. In order to produce highquality medical devices that satisfy both customer and regulatory criteria, it highlights the significance of risk management, process control, and continual improvement. A company’s reputation and competitiveness will eventually increase as a result of less product recalls, customer complaints, and returns.
- Enhanced client satisfaction : A significant emphasis is placed on customer satisfaction and focus. Organisations can improve their understanding of client requirements, satisfy their needs and expectations consistently, and effectively handle any feedback or complaints by putting into place a QMS based on this standard. Increased customer satisfaction generates favourable referrals, repeat business, and customer loyalty.
- Cost effectiveness and efficiency : It encourages the organisation to implement effective process resource management techniques. Streamlining processes, cutting waste, and allocating resources optimally allow businesses to increase output, save operating expenses, and boost total profitability. Organisations can more efficiently allocate resources by concentrating on areas that present the most risks to product quality and regulatory compliance when they prioritise riskbased decision making.
- International market access : Being certified to ISO 13485 signifies an organization’s dedication to quality and legal compliance, which makes it easier to access foreign markets. The ISO 13485 certification is widely accepted by regulatory bodies and customers globally as proof of a strong quality management system. Acquiring this accreditation can assist companies in reaching a wider audience, drawing in new clients, and taking advantage of international commercial prospects.
- Continuous improvement : It promotes the adoption of a continuous improvement culture within organisations, in which systems, products, and processes are routinely assessed and improved in order to provide better results.
Cost Of ISO 13485 Certification In Muscat
The cost of obtaining ISO 13485 certification in Muscat depends on several factors, including the size and complexity of your organization and the specific scope of certification. Factors such as the number of employees, the processes to be audited, and preparatory steps like gap analysis or documentation support can influence the total cost. PopularCert offers tailored, cost-effective ISO 13485 certification solutions designed to meet the unique quality management needs of your medical device business in Muscat. We will guide you through the process and provide details on the cost involved to help you get started on your ISO 13485 Certification journey with PopularCert in Muscat.
Why Choose PopularCert For ISO 13485 Certification In Muscat?
Choose PopularCert for ISO 13485 certification in Muscat to ensure your medical device quality management system complies with international standards and achieves exceptional outcomes. Our experienced consultants will guide you through every stage of the process, from initial gap analysis to successful certification. We help you implement best practices, mitigate risks, and enhance operational efficiency. With PopularCert, you’ll receive tailored support designed to address the specific quality requirements of your medical device organization, ensuring a smooth certification journey and long-term success. Trust us to enhance your quality processes, build stakeholder confidence, and unlock new opportunities with ISO 13485 certification in Muscat.
GET A FREE CONSULTATION NOW
FAQ
What is ISO 13485 and why is it important in Muscat?
ISO 13485 is an international standard for quality management systems in the medical device industry. In Muscat, it’s essential for companies to meet regulatory requirements, ensure product safety, and gain trust from stakeholders and customers.
What is the duration required to obtain ISO 13485 certification?
The time it takes to get ISO 13485 certification can vary depending on your organization’s size and readiness. On average, it may take a few months, with PopularCert helping streamline the process for quicker results.
What are the expenses associated with obtaining ISO 13485 certification?
The cost of ISO 13485 certification depends on factors like the size of your organization and the complexity of your processes. Additional costs may include audits, gap analysis, and documentation support, but PopularCert offers tailored solutions to keep it affordable.
What are the advantages of ISO 13485 for my company?
Obtaining ISO 13485 certification increases your company’s reputation in the marketplace, guarantees legal compliance, and boosts productivity and client happiness.
