ISO 13485 Certification In Riyadh
Get Free Consultation
ISO 13485 Certification in Riyadh supports medical device manufacturers and related organizations in demonstrating their commitment to quality, safety, and regulatory compliance. This internationally recognized standard focuses on establishing a robust quality management system specifically designed for the medical device industry. Achieving ISO 13485 in Riyadh helps organizations meet both local and global regulatory requirements, ensure product consistency, and enhance patient safety. It builds trust with healthcare providers, customers, and stakeholders while boosting market access, operational efficiency, and competitive advantage within Saudi Arabia and beyond.
What is ISO 13485?
ISO 13485 is an international standard that outlines the requirements for a quality management system (QMS) specifically for companies involved in the design, production, installation, and servicing of medical devices.
In simple terms, ISO 13485 helps medical device companies ensure their products are safe, reliable, and meet both customer and regulatory requirements. It focuses on risk management, product traceability, and maintaining strict hygiene and documentation controls throughout the manufacturing process.
This certification is essential for building trust with regulators, healthcare providers, and patients, especially in industries where safety and quality are critical.
Why ISO 13485 Certification is Important in Riyadh.
ISO 13485 Certification is essential for medical device manufacturers and suppliers in Riyadh, ensuring that they meet international standards for quality management systems (QMS). This certification demonstrates the organization’s ability to design, develop, produce, and maintain medical devices that meet regulatory requirements and ensure patient safety.
In Riyadh, where healthcare is a growing sector, ISO 13485 is critical for compliance with both local regulations and international standards. Achieving this certification builds trust with clients, regulatory authorities, and healthcare professionals by assuring the safety, efficacy, and quality of medical devices.
How to Get ISO 13485 Certification In Riyadh?
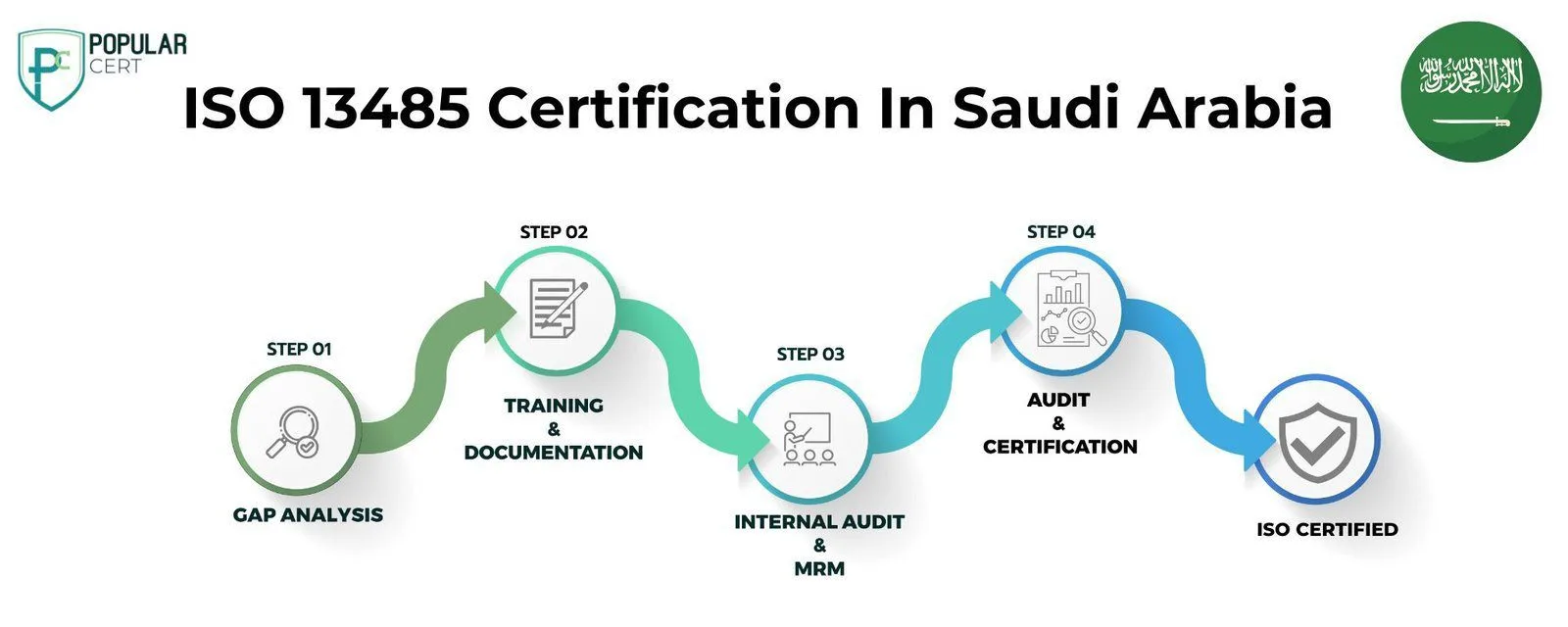
Process to Get ISO 13485 Certification In Riyadh
Consultation and Gap Analysis
Conduct a detailed analysis to identify gaps between current processes and ISO 13485 standards. Familiarize yourself with the ISO 13485 standard and its specific requirements for medical devices.
Planning, Documentation, and Policy Development
Develop or update quality management system documentation, including policies, procedures, and records.
Training and Awareness
Train staff on ISO 13485 principles and their roles in maintaining compliance.
Internal Audit and Management Review
Conduct internal audits to evaluate system effectiveness and identify areas for improvement. Hold management reviews to assess audit findings and the system’s overall performance.
External Certification Audit and Certification
Staying ahead of regulatory requirements is crucial in the medical industry. ISO 13485 aids organizations in achieving and maintaining regulatory compliance, reducing the risk of non-compliance issues and associated legal and financial repercussions.
Benefits of ISO 13485 Certification in Riyadh
- Global Recognition and Compliance : Contact Popularcert: Contact Popularcert to discuss your organization’s requirements and understand the ISO 13485 certification process.
- Increased Efficiency : The standard encourages organizations to streamline their processes, reduce inefficiencies, and optimize resource utilization. This not only enhances productivity but also contributes to overall cost-effectiveness.
- Enhanced Productivity : Popularcert will conduct interanalysis to identify areas where your organization needs improvement to meet ISO 13485 requirements.
- Enhanced Customer Satisfaction :Ensuring the consistent quality of medical devices helps organizations enhance customer satisfaction, build trust, and establish long-lasting relationships with healthcare professionals and end-users.
- Regulatory Compliance : Staying ahead of regulatory requirements is crucial in the medical industry. ISO 13485 aids organizations in achieving and maintaining regulatory compliance, reducing the risk of non-compliance issues and associated legal and financial repercussions.
- Attractive to Investors and Partners : ISO certification makes companies more appealing to investors and business partners by demonstrating a commitment to quality, risk management, and sustainability. This can open doors to new business opportunities, joint ventures, and increased investment.
Types Of ISO Certification In Riyadh
Get Free Consultation
Our Clients


















Why do you need ISO 13485 Certification in Riyadh?
ISO 13485 certification is crucial for medical device manufacturers and suppliers in Riyadh to demonstrate their commitment to quality and regulatory compliance. This certification ensures that organizations design, manufacture, and maintain medical devices that meet international standards for safety, effectiveness, and performance. With the healthcare sector growing rapidly in Riyadh, aligning with global standards is vital for gaining market trust and accessing new business opportunities.
ISO 13485 certification is particularly important as it helps organizations comply with stringent local and international regulations, including those set by the Saudi Food and Drug Authority (SFDA). It enhances customer confidence by ensuring that medical devices meet required quality standards, reducing risks associated with product failures and recalls. Additionally, it streamlines processes, improves operational efficiency, and reduces costs associated with non-compliance.
PopularCert provides end-to-end support for ISO 13485 certification in Riyadh, offering services such as gap analysis, training, and audits to ensure smooth certification.
How ISO 13485 certification in Riyadh can help?
- Understand the Value of ISO Certification : Recognize its role in enhancing business credibility, operational efficiency, and alignment with international standards, critical for thriving in a competitive market.
- Align with Vision 2030 Goals : Utilize ISO certification to foster innovation, sustainability, and excellence, supporting Saudi Arabia’s transformative objectives.
-
Choose the Right Certification : Focus on key standards such as:
- ISO 9001: Quality Management
- ISO 14001: Environmental Management
- ISO 45001: Occupational Health and Safety
- ISO 27001: Information Security
- ISO 50001: Energy Management
- Expand Internationally : Use certification to access global markets, build partnerships, and confidently grow your business footprint.
- Partner with Experts : Collaborate with professionals to simplify the certification process, ensuring compliance and unlocking sustainable growth opportunities.
Why Choose PopularCert For ISO 13485 Certification in Riyadh?
PopularCert provides comprehensive ISO 13485 certification services in Riyadh, ensuring a hassle-free certification process. Our experienced team of advisors will guide you through every step, from internal analysis to validation. We specialize in offering competitive cost for ISO 13485 certification in riyadh, helping you achieve compliance without exceeding your budget, while ensuring high-quality services.
Cost of ISO 13485 Certification in Riyadh
The cost of ISO 13485 certification in Riyadh depends on factors like the organization’s size, scope of certification, and current compliance level. Larger or more complex businesses and those certifying multiple products or locations typically face higher expenses. Costs also include employee training and certification body fees for audits and registration.
PopularCert offers tailored, cost-effective solutions to simplify your ISO 13485 certification process in Riyadh.
GET A FREE CONSULTATION NOW
FAQ
What is ISO 13485 Certification?
ISO 13485 certification is an international standard for quality management systems specifically for the medical device industry. It ensures that organizations consistently produce safe, effective, and compliant medical devices by meeting regulatory requirements and adhering to rigorous quality control processes.
How organizations in Riyadh can achieve ISO 13485 certification?
Organizations in Riyadh can achieve ISO 13485 certification by implementing a robust quality management system, conducting internal audits, training staff, ensuring regulatory compliance, and undergoing certification body audits. PopularCert provides support with gap analysis, documentation, audits, and certification processes.
Benefits of ISO 13485 certification in Riyadh?
ISO 13485 certification in Riyadh enhances product quality, ensures regulatory compliance, and boosts customer confidence. It enables access to global markets, improves operational efficiency, reduces risks, and ensures medical device safety, meeting both local and international healthcare standards.
