ISO 17025 Certification in Riyadh
Get Free Consultation
ISO 17025 Certification in Riyadh bears significance for testing and calibration laboratories wanting to showcase their technical skills and international benchmarking. This standard is accepted internationally and demonstrates how laboratories build trust to regulators, clients, and industry partners with consistent and reliable results.
ISO 17025 is critical in key sectors such as oil and gas, healthcare, construction, and manufacturing, supporting Saudi Vision 2030 objective on quality, innovation, and competitiveness.
PopularCert provides holistic ISO 17025 certification services in Riyadh. We perform gap analysis, documentation, staff training, implementation, internal audits to aid laboratories with our expert team. Your journey towards certification is simplified with our comprehensive solutions while ensuring compliance and operational excellence.
Laboratories based in Riyadh that obtained ISO 17025 certification could improve credibility, fulfill regulatory standards, and access international contracts and markets. PopularCert is the ideal choice to aid in the strengthening of quality systems for sustainable growth.
Why do you need ISO 17025 Certification in Riyadh?
Obtaining ISO 17025 Certification Riyadh is important for testing and calibration laboratories which wish to operate under a rigorous system of maintained technical proficiency, proper measurement, and international benchmarks. It is crucial for laboratories in industries like oil and gas, healthcare, construction, and manufacturing because this certification helps certify labs that they can always achieve trustable and valid results.
Riyadh is a fast growing industrial city and Saudi Vision 2030 places importance on development focused on quality. ISO 17025 supports the enhancement of the national quality system, especially with regards to certified quality infrastructure. Labs with such credentials have a decisive advantage because they can compete withand align with benchmarked counterparts in other countries, meet local and international stipulations, and instill confidence in their customers and stakeholders.
In Riyadh, these labs become sought after in the global market because they offer unmatched innovation and foster sustainable partnerships. Their credibility boosts significantly with the attainment of ISO 17025, thus transforming them into trusted collaborators for projects worldwide.
How to Get ISO 17025 Certification in Riyadh?
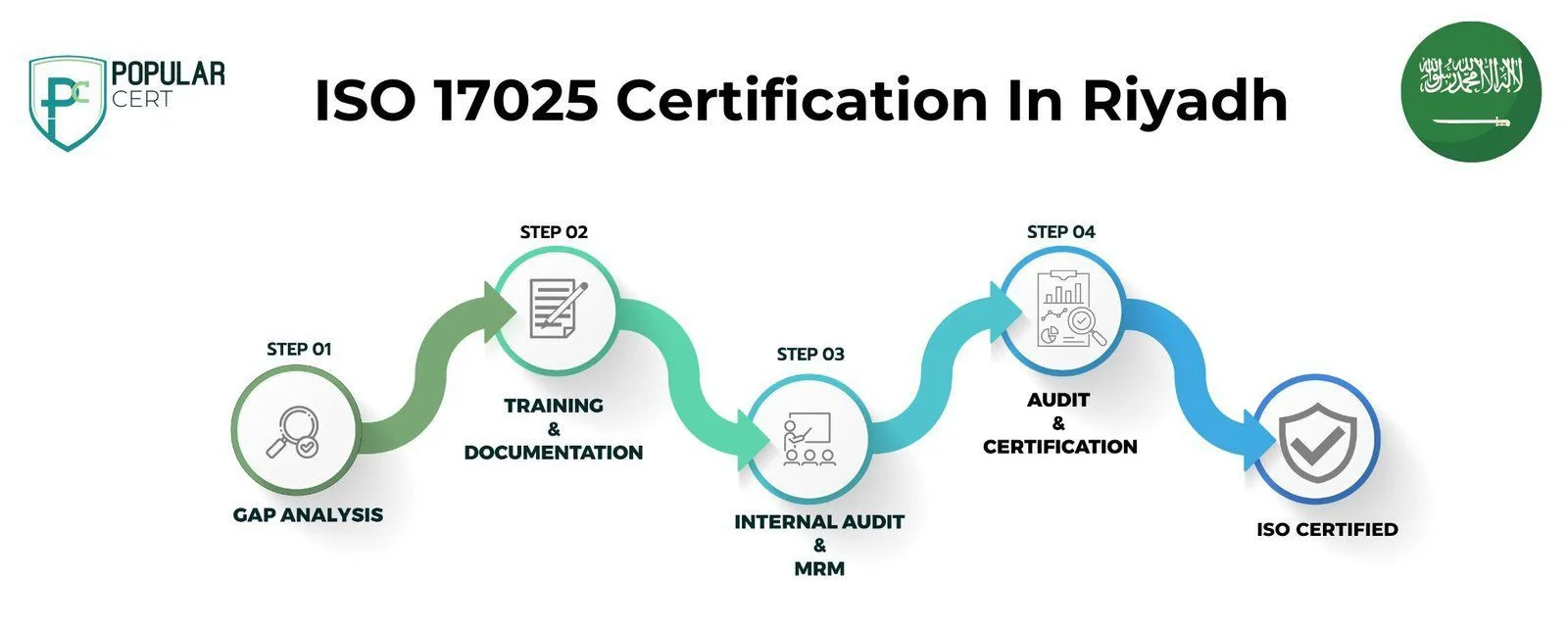
Process to Get ISO 17025 Certification In Riyadh
Consultation and Gap Analysis
PopularCert’s experts assess your organization's specific requirements and existing laboratory management systems. We conduct a detailed gap analysis to identify areas requiring improvement to meet ISO 17025 standards.
Planning, Documentation, and Policy Development
Based on the gap analysis results, we develop a tailored implementation plan, outline resource requirements, and assist in creating essential policies and documentation. These are carefully integrated into your current laboratory operations to ensure smooth alignment with ISO 17025 requirements.
Training and Awareness
We provide comprehensive training to ensure your laboratory staff fully understand ISO 17025 requirements and their roles in maintaining an effective laboratory management system.
Internal Audit and Management Review
Our team conducts internal audits to assess the effectiveness of your laboratory management system, addressing any non-conformities. A management review is performed to ensure the system aligns with both ISO 17025 standards and your organization's quality objectives.
External Certification Audit and Certification
Upon successful completion of an external audit by an accredited certification body, your laboratory will be awarded the ISO 17025 certification. This achievement demonstrates your commitment to reliable testing, quality assurance, and international compliance, enhancing both credibility and customer trust.
Benefits of ISO 17025 Certification in Riyadh
- Enhanced Credibility: Demonstrates your laboratory's commitment to producing accurate and reliable results, building trust with clients and stakeholders.
- Global Recognition: Aligns your laboratory with international standards, increasing its reputation and acceptance worldwide.
- Improved Efficiency: Streamlines processes, reduces errors, and enhances the overall efficiency of your laboratory operations.
- Regulatory Compliance: Ensures your laboratory meets industry regulations and requirements, avoiding non-compliance issues.
- Customer Confidence: Builds trust by guaranteeing high-quality and precise testing or calibration services.
- Competitive Advantage: Helps your lab stand out in Riyadh's competitive market, attracting more clients and business opportunities.
Case Study: Raising the Standard — How a Riyadh Calibration Lab Achieved ISO 17025 Certification with PopularCert
Client: PrecisionCal Labs, Riyadh
Industry: Calibration & Testing for Oil & Gas Equipment
Service Provided: ISO 17025 Certification Consulting
Duration: 5 months
The Challenge
PrecisionCal Labs is a mid-sized calibration laboratory located in Riyadh. The calibration laboratory was not able to capture value-added contracts with oil and gas companies. Though they had sophisticated equipment and capable personnel, they did not have ISO 17025 accreditation, which was a mandatory requirement for most clients.
Moreover, the laboratory struggled with managing documents and other calibrations such as: compliance control, assessment of risks, and traceability in methodologies—all essential in the ISO/IEC 17025:2017 standard. The lab’s increasing competition needed a dependable partner to help them achieve certification within a short timeframe and with utmost confidence.
Our Approach
We provided PrecisionCal with an extensive inner gap analysis looking into their sharp practices relative to the ISO 17025 standard precisioncal. From the results, we created a step-by-step strategy that included:
- Building a tailor-fit quality manual and standard operating procedures.
- Incorporating uncertainty measurement models.
- Teaching corrective action internal audits.
- Setting up defined positions and a skill chart grid.
- Aligning accruement forecasts with international standards.
Final preparatory sessions for accreditation audits
The Result
In 5 months, PrecisionCal received ISO 17025 Certification from an internationally recognized body. Resulting in:
- Acquisition of two new calibration contracts from oil field service companies located in Riyadh.
- Enhanced turnaround time for reporting by 20%
- Documented staff competence aligned to industry benchmarks.
- Increases in market visibility due to inclusion on the Saudi Quality Council’s vendor list.
Business Impact
Achieving the Saudi Vision 2030 objectives for industrial metrology and quality infrastructure indicators offered new avenues for PrecisionCal with the globally accepted ISO 17025 accreditation. This added credibility allowed expansion of laboratory services to clients in the healthcare and manufacturing sectors, diversifying revenue streams beyond oil and gas.
Key Takeaway
“Prior to obtaining ISO 17025, we could not capture the interest of any major lab clients. With PopularCert, we received much more than a certificate; we were able to establish a culture that prioritizes quality first.”
– Lab Director, PrecisionCal Labs
Types Of ISO Certification In Riyadh
Get Free Consultation
Our Clients


















Cost of ISO 17025 Certification in Riyadh
The price for obtaining ISO 17025 certification in Riyadh ranges between some important factors like the laboratory’s size, the range of testing or calibration services offered, and the degree of pre-existing alignment with ISO/IEC 17025:2017 standards. Costs may also depend on whether an initial gap analysis is performed, documentation development, or staff training.
We at PopularCert have flexible certification solutions that are tailored to suit distinct operational requirements and budgetary constraints. Our consulting team ensures that the certification process is seamless and your lab’s normal operations are not affected.
Why Choose PopularCert for ISO 17025 Certification
In Riyadh, PopularCert has tailored solutions for testing and calibration laboratories such as ISO 17025 consultancy that ensures effortless and effective attainment of certification. We work to achieve full compliance with all the requirements of ISO/IEC 17025:2017 including bespoke solutions to ensure that your operations are not disrupted. PopularCert provide complete support, from gap analysis and documentation to internal audits and preparation for accreditation with our seasoned auditors and technical specialists. Our prompt response, compliance with global quality benchmarks, and cost-effective packages set us apart.
Contact PopularCert today to schedule your free consultation or take the first step towards obtaining ISO 17025 certification in Riyadh
GET A FREE CONSULTATION NOW
FAQ
What is ISO 17025 certification?
ISO 17025 is an international standard for testing and calibration laboratories. It specifies the requirements for competence, impartiality, and consistent laboratory operations, ensuring the accuracy and reliability of test results.
Why is ISO 17025 certification important for laboratories in Riyadh?
ISO 17025 certification helps laboratories demonstrate technical competence, ensures the accuracy of test results, improves customer confidence, and enhances global market access.
How do I apply for ISO 17025 certification in Riyadh?
You can apply by partnering with professional consultants like PopularCert, who will guide you through documentation, training, and the certification audit process.
How long does it take to get ISO 17025 certified?
The timeline typically ranges from 3 to 6 months, depending on the laboratory’s readiness and existing quality management practices.
How much does ISO 17025 certification cost in Riyadh?
The cost varies depending on factors such as the size of the laboratory, complexity of testing, and the certification body chosen. PopularCert offers competitive, cost-effective solutions.
