The Importance of ISO 13485 Certification for Tanzania's Medical Device Industry

In Tanzania’s rapidly growing medical device industry, ensuring product quality and safety is more critical than ever. For organizations striving to meet international standards, ISO 13485 certification offers a robust framework to establish an effective Quality Management System (QMS). This certification not only enhances operational efficiency and risk management but also builds credibility, opening doors to both local and global markets.
By adhering to ISO 13485 Certification in Tanzania medical device manufacturers can elevate their competitiveness, instil trust among healthcare providers and patients, and ensure compliance with stringent regulatory requirements. In a sector where quality and safety are non-negotiable, ISO 13485 certification is a strategic investment that paves the way for sustainable growth and success.
What iso ISO 13485 Certification?
ISO 13485 is an international standard which governs the production of medical devices. This standard lays down requirements for the quality management system (QMS) applicable to organizations involved in the design, manufacturing, and marketing of medical devices. For practitioners in Tanzania, this certification is not just a status symbol, but a guarantee of the fact that patients can trust you to provide them with the best possible care while ensuring their safety at all times.
What iso ISO 13485 Certification?
ISO 13485 is an international standard which governs the production of medical devices. This standard lays down requirements for the quality management system (QMS) applicable to organizations involved in the design, manufacturing, and marketing of medical devices. For practitioners in Tanzania, this certification is not just a status symbol, but a guarantee of the fact that patients can trust you to provide them with the best possible care while ensuring their safety at all times.
Benefits of ISO 13485 Certification in Tanzania:
- Increases Patient Safety: The main objective of ISO 13485 is managing medical devices and ensuring their safety as well as usability. Following this standard enables healthcare providers in Tanzania to manage the risks related to deficient or hazardous equipment and safeguard the patients.
- Improves Reputation: Trust is one of the most important components in a competitive healthcare marketplace. The ISO 13485 certification shows clients, associates, and governing bodies that the organization operates within internationally acceptable standards of quality. This improves your reputation and offers you a competitive edge.
- Improves Reputation: Trust is one of the most important components in a competitive healthcare marketplace. The ISO 13485 certification shows clients, associates, and governing bodies that the organization operates within internationally acceptable standards of quality. This improves your reputation and offers you a competitive edge.
- Allows Better Resource Management: The certification process itself requires the organization to improve their processes including streamlining activities, cutting back on waste, and becoming more efficient. Thereby, time and resources are spent more wisely and the level of service given to patients is improved.
- Risk Mitigation: Healthcare regulations continue tightening across the globe. For healthcare providers in Tanzania, ISO 13485 helps mitigate violations of both local regulations and international sanctions, thereby avoiding costly penalties and legal problems.
Why is ISO 13485 Important in Tanzania?
In Tanzania’s healthcare sector, where regulations are stringent and the demand for quality medical devices is growing, ISO 13485 certification is a game-changer. Here’s why it matters:
- Regulatory Compliance: Ensures that medical devices meet Tanzania’s health and safety regulations, reducing the risk of non-compliance and legal issues.
- Quality Assurance: Enhances the consistency and reliability of medical products, ensuring they meet global quality standards.
- Market Access: Facilitates easier entry into local and international markets by demonstrating adherence to recognized standards.
- Customer Confidence: Builds trust among healthcare providers and patients by ensuring product safety and effectiveness.
- Operational Efficiency: Streamlines manufacturing and quality control processes, minimizing errors and reducing costs.
- Competitive Advantage: Differentiates certified companies from uncertified competitors, strengthening their position in the industry.
Which Vendors in Tanzania Should Obtain ISO 13485 Certification?
ISO 13485 certification is essential for a wide range of vendors involved in Tanzania’s Medical Device industry. Key players who should pursue this certification include:
- Medical Device Manufacturers: Companies designing and producing medical devices must ensure their products meet international quality and safety standards.
- Suppliers of Medical Components and Materials: Vendors providing essential parts or raw materials for medical devices play a critical role in the supply chain and must adhere to stringent quality management practices.
- Contract Manufacturing Organizations (CMOs): Third-party manufacturers producing medical devices on behalf of other companies need certification to demonstrate compliance with regulatory requirements.
- Distributors and Importers of Medical Devices: Entities responsible for bringing medical devices into the Tanzanian market must ensure the products they handle comply with recognized quality standards.
Types Of Certification
- ISO Certification
- ISO 9001 Certification
- ISO 14001 Certification
- ISO 45001 Certification
- ISO 22000 Certification
- ISO 27001 Certification
- ISO 17025 Certification
- ISO 13485 Certification
- ISO 20000-1 Certification
- ISO 22301 Certification
- ISO 50001 Certification
- ISO 37001 Certification
- IATF 16949 Certification
- ISO 29001 Certification
- ISO 31000 Certification
- ISO 20121 Certification
- ISO 10002 Certification
- ISO 41001 Certification
Get Free Consultation
Our Clients


















Cost of ISO 13485 Certification in Tanzania:
The cost of obtaining ISO 13485 certification varies depending on factors such as the size of your organization, the complexity of your processes, and the scope of certification. While initial investments are required, the long-term benefits such as improved product quality, regulatory compliance, and expanded market access far outweigh the costs.
Partnering with Popularcert ensures a seamless certification process, backed by expert guidance and tailored solutions. Our comprehensive support in consulting, training, and audits enables businesses to achieve certification efficiently while optimizing operational performance.
Why PopularCert is the best for ISO 13485 Certification in Tanzania?
Navigating the ISO 13485 certification process can be complex, but Popularcert is here to help. As a trusted certification service provider, Popularcert offers expert guidance and tailored solutions to ensure your organization meets all requirements efficiently.
- Expert Guidance: Our seasoned professionals provide personalized support throughout the certification journey.
- Customized Solutions: We tailor our services to align with your organization’s unique needs and industry standards.
- Enhanced Credibility: Certification through Popularcert boosts stakeholder confidence and market reputation.
- Operational Excellence: Implementing best practices leads to improved processes and efficiency.
- Competitive Advantage: Certification sets your company apart, attracting new business opportunities.
Partnering with experts like Popularcert can facilitate a seamless certification journey, positioning companies for sustained success in the competitive medical device industry. By investing in ISO 13485 certification, Tanzanian businesses can ensure operational excellence, build long-term credibility, and achieve sustainable growth in the global healthcare market.
GET A FREE CONSULTATION NOW
FAQ
What is ISO 13485 certification, and why is it important for medical device companies in Tanzania?
ISO 13485 is an internationally recognized standard that outlines the requirements for a Quality Management System (QMS) specific to the medical device industry. For Tanzanian medical device companies, obtaining ISO 13485 certification demonstrates a commitment to producing safe and effective products, ensuring compliance with both local and international regulatory requirements. This certification enhances credibility, facilitates market access, and fosters customer trust.
What are the key steps involved in obtaining ISO 13485 certification in Tanzania?
The process of obtaining ISO 13485 certification in Tanzania involves several critical steps:
- Developing a QMS: Establish processes and procedures that align with ISO 13485 standards to ensure product quality and safety.
- Conducting Internal Audits: Regularly assess the effectiveness of the QMS and identify areas for improvement.
- Management Review: Ensure top management evaluates the QMS for suitability and effectiveness.
- Corrective Actions: Address any identified non-conformities to prevent recurrence.
- External Audit: Engage a recognized certification body to perform a comprehensive audit of the QMS.
- Certification: Upon successful completion of the external audit, the organization is awarded ISO 13485 certification.
Partnering with experienced consultants, such as Popularcert, can provide tailored guidance throughout this process, ensuring a seamless certification journey.
How does ISO 13485 certification benefit medical device manufacturers in Tanzania?
Achieving ISO 13485 certification offers numerous advantages to Tanzanian medical device manufacturers, including:
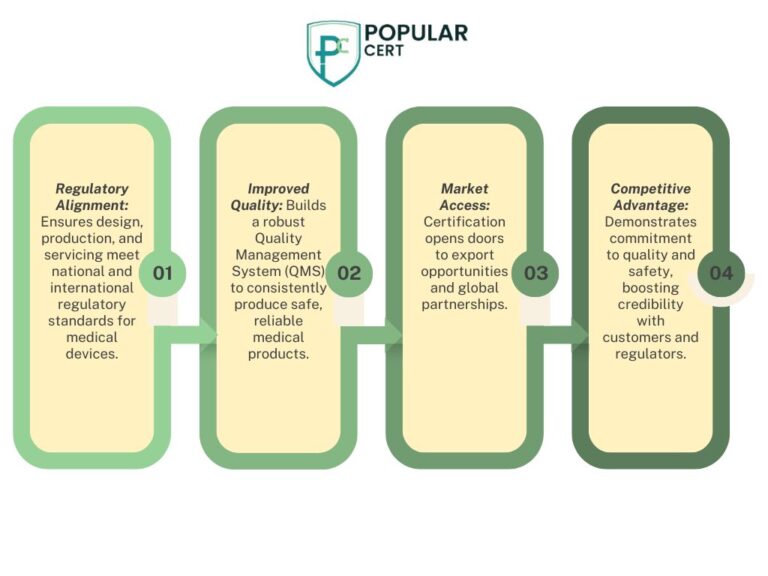
- Regulatory Compliance: Ensures products meet national and international regulatory standards, reducing the risk of non-compliance.
- Market Access: Facilitates entry into global markets where ISO 13485 certification is a prerequisite.
- Enhanced Credibility: Demonstrates a commitment to quality and safety, building trust with customers and stakeholders.
- Operational Efficiency: Streamlines processes, leading to reduced errors and increased productivity.
- Risk Management: Promotes proactive identification and mitigation of potential risks associated with medical device production.
