ISO 13485 Implementation in Oman’s Healthcare Sector: A QMS Guide for Medical Devices
Oman’s healthcare vision for 2040 is driving rapid growth in hospitals, clinics, and new technologies, and that means every product used must be safe and reliable. To keep pace, companies in the medical sector are turning to the world’s most respected quality seal ISO 13485 Certification because it shows they have a mature Quality Management System (QMS) in place.
Whether you make devices, import lab equipment, or run a clinic that uses advanced imaging machines, adopting this standard proves your work meets every relevant rule and the expectations of every customer. In the pages ahead we explain the step-by-step path for Omani firms and show how teaming up with a skilled partner like Popularcert makes that journey quicker and less stressful.
What is ISO 13485?
ISO 13485:2016 lays out clear, detailed criteria for a QMS dedicated solely to medical devices. While it shares many ideas with ISO 9001, it goes further by adding requirements on risk controls, regulatory audits, post-market surveillance, and documentation at every point of the product life cycle.
When an organization earns the ISO 13485 badge, it signals to regulators and the public that its processes deliver safe, effective products from design through delivery-and that patient safety is baked into every decision.
Why ISO 13485 Matters in Oman’s Healthcare Landscape
Oman’s healthcare sector is changing fast, driven by bigger public budgets, foreign partnerships, and a thirst for cutting-edge tech. In this busy landscape, ISO 13485 gives you:
- full MoH compliance, keeping the local regulators happy;
- a ticket to global markets such as Europe, the U.S., and the wider GCC;
- stronger patient safety and more dependable products;
- a better brand image that opens more procurement doors.
For device makers and importers in Oman, ISO 13485 has moved from nice-to-have to must-have for winning tenders and getting products registered.
Key Benefits of ISO 13485 Certification
- Regulatory Alignment: the standard aligns with CE, U.S. FDA, and MoH rules, so you tick several boxes at once.
- Operational Efficiency: a solid QMS means fewer errors, less downtime, and lower recall costs.
- Market Access: certification clears the way to global sales and local hospital tenders that ask for a formal QMS.
- Increased Trust: having the badge raises confidence with regulators, suppliers, clinics, and patients at home and abroad.
Core Requirements of ISO 13485 QMS
-
Quality Policy and Documentation:
1. Establish clear quality objectives and a guiding policy.
2. Write documented procedures, SOPs, and an easily usable quality manual.
3. Apply risk-based thinking to every procedure and record. -
Design and Development Controls:
1. Set firm controls for design validation and verification.
2. Manage design changes with clear documentation and traceability. -
Supplier Management:
1. Evaluate and formally approve suppliers deemed critical.
2. Perform regular audits and review supplier performance. -
Production and Process Control:
1. Validate sterilization processes where required.
2. Control the environment and actively prevent contamination. -
Monitoring and Improvement:
1. Run internal audits and act on CAPA findings.
2. Track management reviews alongside customer feedback.
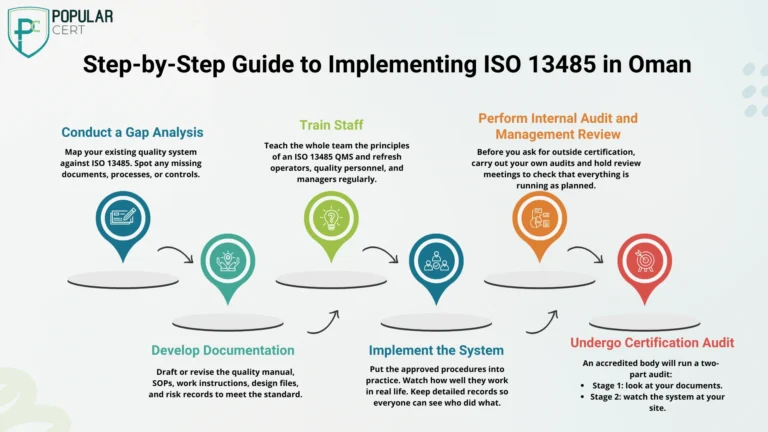
Step-by-Step Guide to Implementing ISO 13485 in Oman
Types Of Certification
- ISO Certification
- ISO 9001 Certification
- ISO 14001 Certification
- ISO 45001 Certification
- ISO 22000 Certification
- ISO 27001 Certification
- ISO 17025 Certification
- ISO 13485 Certification
- ISO 20000-1 Certification
- ISO 22301 Certification
- ISO 50001 Certification
- ISO 37001 Certification
- IATF 16949 Certification
- ISO 29001 Certification
- ISO 31000 Certification
- ISO 20121 Certification
- ISO 10002 Certification
- ISO 41001 Certification
Get Free Consultation
Our Clients


















ISO 13485 in Oman: Who Needs It?
In Oman, the following groups gain value from-or must hold-ISO 13485:
- Manufacturers and assemblers of medical devices.
- Importers and distributors of foreign products.
- Hospitals and clinics with high-risk equipment.
- Diagnostic labs and digital-health start-ups.
- Firms that contract sterilization or packaging.
Why Choose Popularcert for ISO 13485 Certification in Oman?
Rolling out ISO 13485 can be technical, and it usually takes longer than expected. That is why Popularcert, Oman’s leading certification-consultancy firm, steps in as your trusted guide.
What Popularcert Offers:
- Gap analysis and complete QMS documents.
- Staff training and audit-readiness plans.
- Hands-on support during every audit stage.
- Clear advice to keep you compliant after certification.
With years of work in health care, biotech and medical devices, Popularcert makes sure your path to ISO 13485 is smooth, fast and fully meets Omani rules.
Conclusion
Whether you are rolling out a brand-new medical device, bringing in international products, or improving how your hospital tracks its tools, ISO 13485 can make-or-break your efforts in Oman’s health market. The standard locks in compliance, proves safety, and boosts your presence worldwide, helping you stay ahead in an industry that never stops changing.
Avoid putting your reputation, market access, or patient lives on the line. Let Popularcert steer you through QMS design, clear paperwork, hands-on training, and smooth certification. With years of success in Oman and the wider Gulf, we show you how to adopt ISO 13485 Certification with the confidence and clarity that really matter.
GET A FREE CONSULTATION NOW
FAQs
Is ISO 13485 certification mandatory in Oman?
Certification is not legally compulsory, but the Ministry of Health typically asks for it when medical devices are registered, and most public tenders or export deals expect it too.
How long does it take to implement ISO 13485 in Oman?
Most companies finish the basics in 3 to 6 months, though larger or more complex businesses, and those with little existing documentation, may need a bit longer.
What’s the difference between ISO 9001 and ISO 13485?
While ISO 9001 sets general quality rules, ISO 13485 digs into medical device risks, regulatory steps, and every stage of the product life cycle, making it stricter and more tailored to the sector.
Can a company without in-house QA staff still get certified?
Absolutely. A good consultancy like Popularcert can write documents, train staff, and build the system either alone or side by side with the company’s own people.
How much does ISO 13485 certification cost in Oman?
Fees depend on the firm’s size and the number of products; as a rough guide, planning, auditing and the final certificate together usually cost between 2000 and 8000 OMR.
