GMP Certified in the Philippines: What It Means & Why It Matters for Manufacturers

Introduction
In the present, product quality and customer safety are essential to meeting industry standards. For businesses in the food, pharmaceutical, cosmetic, and health sectors, Trust, partnershipSSL, and market access both locally and globally rely on GMP certification in the Philippines.
This blog explains the meaning of “GMP certified,” its benefits, the certification steps within the Philippines, and the expert support Popularcert provides.
What Does “GMP Certified” Mean?
GMP certified companies have passed strict inspections and documentation demands, which guarantees compliance with the controlled production and GMP documentation requirements.
Having GMP certification also means the following:
- GMP certified facilities in the Philippines are marked byThese include:
- A clean and hygienic controlled atmosphere
- Staff that are properly trained
- Quality control systems that can be relied on
The Philippine FDA is the regulatory body responsible for the enforcement of GMP compliance with the controlled products and is aligned with other international practices like WHO GMP and ISO.
Why Is GMP Certification Important in the Philippines?
The Philippines has a booming agro-industrial base and a thriving food and health-related business sector, including firms ready to export. With heightened consumer scrutiny and tighter regulations, obtaining a GMP certification no longer is optional—it has become essential for business.
Here’s why it is important:
-
Consumer Health And Safety:
Ensures your products are safe and do not pose any health risks and are free from contamination. -
Market Access:
Multiple locals and international stores have a requirement of certified GMP accredited documents as a prerequisite to do business with them. -
Regulatory Compliance:
Avoids penalties, recalls, and enforcement actions from FDA Philippines. -
Business Credibility:
Demonstrates that you operate your business using the best globally accepted quality and safety standards for firms in your industry.
Industries That Require GMP Certification
Types Of Certification
- ISO Certification
- ISO 9001 Certification
- ISO 14001 Certification
- ISO 45001 Certification
- ISO 22000 Certification
- ISO 27001 Certification
- ISO 17025 Certification
- ISO 13485 Certification
- ISO 20000-1 Certification
- ISO 22301 Certification
- ISO 50001 Certification
- ISO 37001 Certification
- IATF 16949 Certification
- ISO 29001 Certification
- ISO 31000 Certification
- ISO 20121 Certification
- ISO 10002 Certification
- ISO 41001 Certification
Get Free Consultation
Our Clients


















Various industries in the Philippines have an interest in, or need to meet, GMP compliance:
- Food and Beverage Industry: Need to comply with hygiene and shelf life requirements.
- Pharmaceutical Industry: Required to provide safety and efficacy of the drugs.
- Cosmetics And Skincare Companies: Product contamination and allergic reactions should be avoided.
- Manufacturers of Medical Devices: Must have precision and sterility during manufacturing.
- Nutraceutical Bulk Herbal Capsules: Must comply with export and labeling regulations.
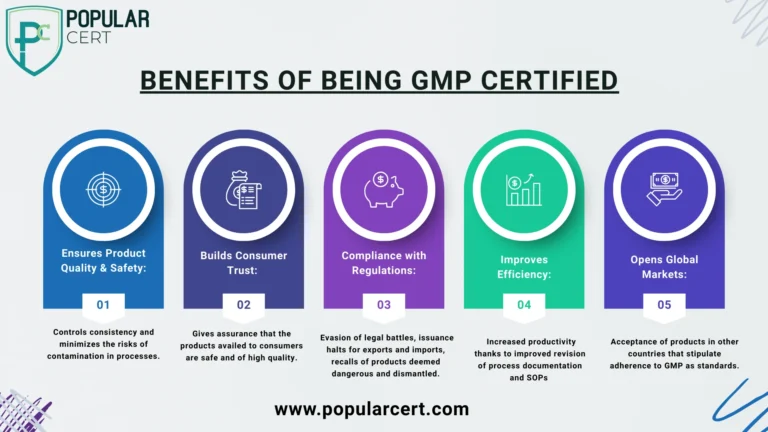
Benefits of Being GMP Certified
Strategically and operationally, there are benefits that come with the decision to acquire a GMP certification:
GMP Certification Requirements in the Philippines
Strategically, there are operational advantages that come with achieving GMP Certification:
Documented Procedures: Creation of SOPs for all crucial operations.
- Facility Layout: Spatial allocation for storing raw materials, processing, and packaging of the products must be clearly engraved.
- Sanitation: Sanitary restrictions and protective wear must be observed and enforced.
- Equipment Maintenance: Scheduled dates for servicing machines and calibration must be put in place.
- Pest Control: Plans aimed at averting pest interference must be put down in documents.
- Plans: Continuous education revolving around GMP for employees is to be documented.
Steps to Become GMP Certified in the Philippines
1.Preliminary Gap evaluation
Before applying, complete a self-assessment or contract Popularcert to:
- Evaluate your operational processes
- Determine what needs to change
- Bring your business operations to comply with GMP benchmarks.
2. Documentation and Training
SOPs, along with other documents, should be created or modified to include:
- Sanitation schedules
- Quality management documentation
- In-house GMP training for all personnel
3. Application to FDA or Certifier
Prepare documents for both:
- The Philippine FDA for regulated products; and
- Book an audit with a registered certifying agency for export purposes.
4. On-site Audit and Certification
During the audit, the inspectors shall:
- Evaluate documents
- Conduct facility walkthroughs
- Interview employees
- Assess compliance with documented procedures.
If you pass the audit, you’ll receive your GMP certificate which is usually valid for 1-3 years.
Who Can Help You Get GMP Certified in the Philippines?
Navigating through the GMP process can be overwhelming for small to medium enterprises. That’s where Popularcert comes in. Popularcert is a trusted consulting firm offering end-to-end GMP certification support in the Philippines. Their services include:
- Gap analysis and readiness assessment
- SOP formulation and compliance documentation
- Training programs
- Internal audits
- Assistance during FDA or third-party inspections
Why Choose Popularcert for Your GMP Certification?
Philippine manufacturers have placed their trust in Popularcert for the following reasons:
- Industry Knowledge: Many years working in the food, pharmaceutical, cosmetic, and supplement industries
- Comprehensive Service: From first consultation to post-certification surveillance
- Know-Local: Strong grasp of the FDA Philippines requirements and their approval timelines
- International Scope: Assists in reaching international markets or meeting GMP requirements of other countries.
With Popularcert, certification is just one of the many things we offer. Effective strategies and peace of mind is guaranteed throughout the entire process.
Common Challenges in GMP Certification – And How to Overcome Them
As much as these certifications are important, there are always obstacles to overcome such as:
- Bad Document Control Systems: Missing or incomplete SOPs.
- Non-Compliance of Staff: Training that isn’t enforced or adherence to outdated protocols.
- Inadequate Design of Facilities: Separation of raw materials and finished goods.
- Pest Control Fail Safes: Rely solely on prevent tactics.
With tailored interventions and real-time support, these obstacles become easily manageable.
Popularcert would also go as far as implementing proactive strategies to help you manage these common hurdles.
Maintaining GMP Certification
Certification is a continuous process that requires businesses to:
- Conduct internal audits regularly
- Maintain SOPs and logs
- Onboard new staff and upskill current employees
- Keep track of regulatory updates
- Prepare for surveillance audits and renewals.
Your GMP compliance, or Good Manufacturing Practice compliance, is ensured to stay operational long after your initial approval with the post-certification services offered by Popularcert.
Final Thoughts: Is Your Business Ready to Be GMP Certified?
In the Philippines, a business or company obtaining a GMP Certification is more than just legal compliance. It showcases the business’s commitment to safety and quality, as well as fostering customer satisfaction—a strategic certification to possess. The business is seen as a trusted and reliable manufacturer with a GMP certification, be it food products, cosmetics, pharmaceuticals, or dietary supplements.
Do you need guidance getting started?
Receive personalized guidance through Popolarcet’s tailored consulting, documentation aid, special training sessions, as well as audit and review help.
Get in touch with Popularcert today and claim your free GMP consultation!
GET A FREE CONSULTATION NOW
FAQs
What does “GMP certified” mean for a company?
It means the company follows Good Manufacturing Practices which safeguards the product’s safety, quality, and validity against industry standards.
Is GMP certification mandatory in the Philippines?
Yes. Companies operating in highly regulated industries such as food, drugs, and cosmetics are mandated to comply by the Philippine FDA.
How long does it take to become GMP certified?
Depending on the scope of work and existing preparedness, it could take between 2 to 6 months to receive certification after prep work such as audits and prep work.
Can a startup get GMP certified?
Yes. Early adoption of GMP certification by startups aids in earning consumer trust and enables robust operational frameworks to be built from the start.
How can Popularcert help with GMP certification?
Popularcert aids by conducting a complete gap analysis, developing relevant SOPs, training staff, and preparing for audits, thus guiding them seamlessly towards certification.
