GMP Good Manufacturing Practice: Everything You Need to Know

Introduction
In today’s highly controlled manufacturing landscape, safeguarding product safety, uniformity, and quality is essential — not an option. This is where GMP (Good Manufacturing Practice) in Saudi Arabia comes into play. Be it pharmaceuticals, food and beverages, cosmetics, or even medical devices — adhering to GMP is not just a regulatory obligation, but it is a morally binding requirement.
In this guide, we will discuss What is GMP, importance of GMP compliance, the certification steps, and Popularcert’s role in the compliance roadmap.
What is GMP (Good Manufacturing Practice)?
Good Manufacturing Practice (GMP) is the set of rules and guidelines regulating every stage of the manufacturing process to ensure that products are manufactured and controlled as per the established quality standards. This is enforced by the FDA in the United States, EMA in Europe, and WHO globally.
GMP oversees the entire production cycle starting from raw materials, facility and equipment, to even the cleanliness and training of the personnel. Compliance to GMP guarantees safety, purity, and effectiveness of products.
Why GMP Compliance Matters
1. Protects Health and Safety of a Consumer
GMP helps address potential problems in the production of pharmaceuticals, foods, and cosmetics, such as contamination, mislabeling, and incorrect dosing supervision. These measures are important in safeguarding public health and the consumers.
2. Builds Business Reliability and Confidence
GMP Qualification, as with any standard, increases the loyalty given to the organization by the appropriate sign of commitment to quality. It indicates to the auditors, the regulators, consumers, and buyers that your systems are functioning as required and your products are marketable and safe.
3. Reduces Waste and Improves Efficiency
GMP improves production in a company by avoiding unnecessary gaps in the record-keeping as well as having set procedures to follow and gaps in documentation to fill, averting unnecessary shut downs and recalls, thus improving efficiency.
Key Principles of GMP
GMP, or Good Manufacturing Practice, is built on fundamental principles that collaboratively ensure quality is sustained throughout the entire production process. These are:
-
Sanitation and Hygiene:
Ensured cleanliness of facilities, equipment, and personnel to avert contamination. -
Staff Qualification:
The training and competency of the personnel is essential as all men and women undertaking the fabrication must be fit to do the work. -
Facilities and Equipment:
The systems, equipment, and auxiliaries should be built to guarantee sufficient workflow, cleanliness of the premises, and order. -
Documentation or record-keeping:
All pertinent information and data must be documented without omission, which, together with comprehensive, unambiguous, and verifiable documentation adds value. -
Quality Management:
The personnel and teams must be tasked with appropriate measures like the safe implementation of the process, control, and any necessary correction action. -
Validation and Calibration:
Regular checks of processes and equipment to make certain performance is stable and consistent. -
Complaint Handling and Recalls:
Procedures in place to manage defects or complaints from consumers.
Types Of Certification
- ISO Certification
- ISO 9001 Certification
- ISO 14001 Certification
- ISO 45001 Certification
- ISO 22000 Certification
- ISO 27001 Certification
- ISO 17025 Certification
- ISO 13485 Certification
- ISO 20000-1 Certification
- ISO 22301 Certification
- ISO 50001 Certification
- ISO 37001 Certification
- IATF 16949 Certification
- ISO 29001 Certification
- ISO 31000 Certification
- ISO 20121 Certification
- ISO 10002 Certification
- ISO 41001 Certification
Get Free Consultation
Our Clients


















Industries That Require GMP
- Food and Beverage: GMP helps make certain that food products are produced, packaged, and stored without contamination and in compliance with hygiene standards. It supports ISO 22000, the international standard on food safety management.
- Pharmaceuticals and Biotechnology: GMP is crucial in pharmaceutical manufacturing due to the severe health consequences of even the smallest deviations from standard procedures. Regulatory approval is often dependent on meeting the stringent requirements of GMP.
- Cosmetics and Personal Care: Manufacturers are obligated to guarantee that the products are non-toxic with a consistent formulation and safe packaging. GMP necessitates controls on cross-contamination and formulation errors.
- Medical Devices: Medical devices including surgical and diagnostic equipment are ensured of safety, traceability, and performance though GMP. These are usually in conjunction with ISO 13485 standards.
GMP Certification Process
- Identifying Gaps
The initial step commits to the evaluation of your processes against GMP requirements. This will highlight gaps and help chart a pathway to achieving compliance.
- Creation of Needed Documents
Documents including SOPs, work instructions, quality manuals, and related process controls are developed and standardized to the required SOPs.
- Staff Training and Workforce Implementation
Workers must be trained on GMP protocols to ensure adequate understanding and uniform application. This promotes a quality culture and accountability across the organization.
- Self Audit and Action Plan
Self audits help to correct any gaps in processes before the final audit. This stage smooths readiness for compliance certification.
- Audit Compliance and Certification
The audit compliance certification body performs the audit to GMP compliance standards then issues a compliance certificate for GMP.
Popularcert actively supports and promotes compliance across all stages, ensuring that you are optimally ready for client audits and tailored to client needs.
Challenges in GMP Implementation (and How to Overcome Them)
Challenges to Implementing GMP include the following:
- Business processes are not standardized: Lack of proper documented SOPs and records.
- Ineffective Change Management: Informal practices that are deeply ingrained in a company’s culture tend to be difficult to shift from.
- Maintenance Backlogs: With aging and obsolete equipment, the associated infrastructure may also require updating.
- Intra and Inter Organizational Structures: Supply chain and other third-party participants must also meet GMP criteria.
Potential Solutions include:
- Adopt a stepwise approach and tackle small, measurable changes to processes.
- Add formally structured training of employees and other stakeholders.
Consult Popularcert and other industry experts to aid in the smooth adoption of GMP practices.
GMP and Other Standards: How It Connects
Integrating additional international standards along with GMP improves the organization’s compliance:
- ISO 9001 (Quality Management Systems): Captures the broader QMS, provides additional support to GMP implementation.
- ISO 22000 (Food Safety): Further works alongside GMP for food manufacturers.
- ISO 13485 (Medical Devices): Incorporates GMP within the med-tech sector for regulatory compliance.
With a strategic approach to certification, companies can meet multiple compliance needs.
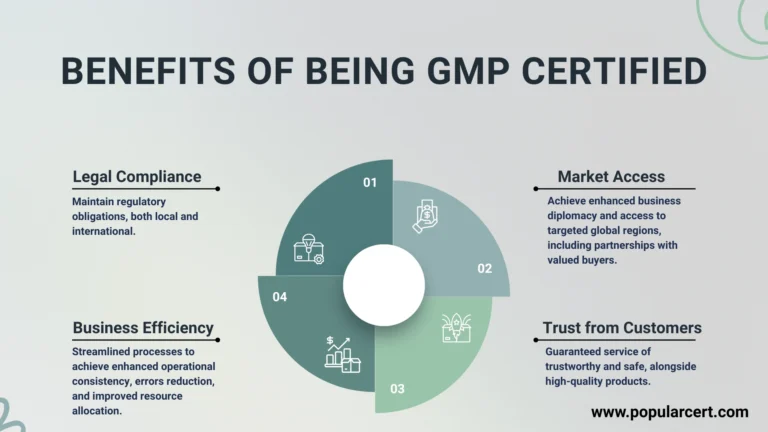
Benefits of Being GMP Certified
How Popularcert Can Help You with GMP
Popularcert is an industry leader in ISO and GMP consulting, servicing the food, pharmaceutical, and manufacturing sectors. Our consultants will assist you with:
- Delphi gap analysis and compliance strategy design
- Assembling SOPs and necessary documentation
- Training, internal audits, and other governance
- Pre-certification audits and ongoing consultancy
When you choose us, the journey to your GMP certification is logical and straightforward. Our consultants work with you to achieve sustained compliance and success beyond certification.
Interested in beginning your GMP journey with us? Reach out today for consultancy and assistance with certification.
Conclusion
GMP is not just another compliance requirement. It is a linchpin of operational efficiency, safety for consumers, and prevalence of the business. Regardless of whether a firm is onboarding a new product into the market, or is in the process of scaling an already-existing business line, good manufacturing practice must be in place.
Focus on growing your business while we support you from the compliance stage to the certified company. We will take care of your systems for the certification while you meet the required quality and safety standards for the GMP benchmarks.
Ready to become certified? We will support you with our tailored services. Reach out to us today.
GET A FREE CONSULTATION NOW
FAQs
What does GMP stand for?
Good Manufacturing GMP stands for Good Manufacturing Practice which is a supported system for all businesses to aid in checking the system as a machinery to check if the goods are always scientifically factored and posted for quality during the production.
Is GMP mandatory?
Yes, GMP is conditional in most of the controlled industries as food goods, medicines or even surgery goods and the controlling country system put in place.
How long does GMP certification take?
This is dependent on the status of your organization’s documenting, processes, and nearness to be ready. While working with Popularcert, the processing is systematized and so easy.
What’s the difference between GMP and ISO 9001?
With ISO 9001, all other management of quality that is to be production managed certified in other industries is broad but the GMP is targeted. The two aid between each other’s weaknesses.
How often is GMP compliance audited?
Most certified and tested facilities are assessed every single year and some in the order of two years. With these, internal checks need to be made often.
