Ultimate Guide to GMP Certification: Good Manufacturing Practices Explained

In today’s tightly regulated world, making safe, reliable, and high-quality products isn’t just a selling point-it is the law. Good Manufacturing Practices or GMP, provide the roadmap for that goal. Whether you work in pharmaceuticals, food, personal care, or medical devices, earning a GMP badge shows you meet rules, earns buyers trust, and keeps your product from losing quality on the shelf.
This guide walks you through what GMP means, why it matters, the steps to certification, and how Popularcert can lead you there without the headaches.
What is GMP (Good Manufacturing Practices)?
- Good Manufacturing Practices are clear, global rules that guard the safety, quality, and effectiveness of what you make. They cover everything-tidy workspaces, careful methods, accurate records, and well-trained staff.
- GMP is mandatory in highly regulated industries like:
- pharmaceuticals food and beverages cosmetics and personal care medical devices nutraceuticals and dietary supplements
- Agencies such as the US FDA, WHO, and Europe’s EMA will not grant market access unless GMP standards are fully met.
Why GMP Compliance is Crucial for Your Business
- Keeps Products Safe and Up to Standard
GMP guides firms so each batch is made the same way, cutting down on dirt, mix-ups, and flaws.
2. Meets the Rules
- Turning a blind eye can bring:
- Product recalls
- Legal fines
- Market bans
- Good practices show you follow local and global laws.
3. Improves Sales Appeal
- GMP seal helps you win:
- Buyer trust
- Clearance for export
- Contract runs.
4. Streamlines Daily Work
- Clear steps and regular checks cut:
- Waste
- Overhead
- Customer complaints
Types Of Certification
- ISO Certification
- ISO 9001 Certification
- ISO 14001 Certification
- ISO 45001 Certification
- ISO 22000 Certification
- ISO 27001 Certification
- ISO 17025 Certification
- ISO 13485 Certification
- ISO 20000-1 Certification
- ISO 22301 Certification
- ISO 50001 Certification
- ISO 37001 Certification
- IATF 16949 Certification
- ISO 29001 Certification
- ISO 31000 Certification
- ISO 20121 Certification
- ISO 10002 Certification
- ISO 41001 Certification
Get Free Consultation
Our Clients


















Key Components of GMP Requirements
Putting Good Manufacturing Practice (GMP) into action means focusing on a few key areas:
-
Facility Design and Hygiene:
Keep the layout clean, tidy, and easy to move through.
Control airflow, block pests, and monitor humidity.
Set clear barriers between raw stock and finished goods -
Equipment Maintenance:
Calibrate, test, and validate machines on a regular schedule.
Clean parts and surfaces with the right chemicals.
Log repairs, inspection dates, and service records -
Personnel Qualifications:
Choose people with the right skills and attitude.
Train them in hygiene, safety, and site rules.
Issue clean uniforms, hats, gloves, and badge controls -
Quality Control & Assurance:
Get SOPs approved before anything leaves the floor.
Test every ingredient and every batch.
Confirm that key steps actually work and stay in control -
Documentation & Record Keeping:
Complete batch logs and hold them securely.
Note every change and why it happened.
Build clear audit trails so nothing gets lost -
Handling of Raw Materials & Finished Products:
Work only with certified suppliers.
Store items at the right temp, humidity, and light.
Label and package goods so customers know what's inside
GMP Certification Process: Step-by-Step
The path to GMP certification usually follows these steps:
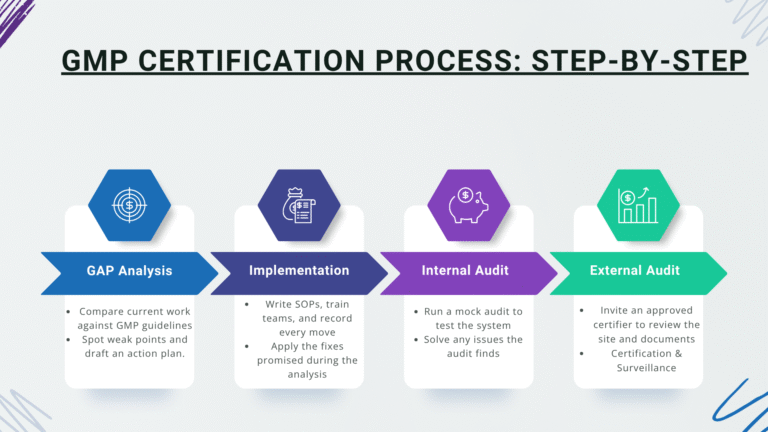
Once granted, the certificate lasts for a set period-usually three years. Each year the body returns for a lighter-scope surveillance audit.
Note: Overall, plan on three to six months if your team is ready.
Global GMP Standards and Certifying Bodies
Many nations and sectors lean on their own GMP rules:
Standard Region Purpose WHO GMP Global Medicines and healthcare goods US FDA cGMP United States Food and pharmaceuticals EU GMP European Union Pharmaceuticals ISO 22716 Global Cosmetics production.
Top Certification Bodies Popularcert SGS TÜV SÜD BSI Intertek
Standard | Region | Purpose |
WHO GMP | Global | Medicines and health-care goods |
US FDA cGMP | United States | Drugs and food |
EU GMP | European | Union Medicines |
ISO 22716 | Global | Cosmetics production |
With specialists around the globe, Popularcert delivers advice tuned to each region so your GMP program meets local standards.
Common Certification Challenges Solved by Popularcert
- Unclear documentation process Ready-made templates plus hands-on editing
- Inexperienced internal teams Industry-tailored training online or on-site
- Shortage of time and resources Step-by-step rollout backed by remote help
- Audit failures In-depth root-cause review and clear corrective steps
Why Choose Popularcert for Your GMP Certification?
- 100% Success Rate on GMP Audits
- Fast-Track Certification Options
- Transparent Pricing-no Last-minute Charges
- 24/7 Consultant Access
- Worldwide Knowledge, Local Action
Conclusion
GMP-Good Manufacturing Practice- isn’t just another box to tick; it’s an active promise to send safe, steady, high-quality goods to every buyer. No matter whether you make drugs, food, cosmetics or medical gear, earning the seal boosts your reputation, cuts risk and opens both local and global shelves.
The road to GMP does not have to swamp you if your team has the right backing. That is exactly the support Popularcert delivers.
As one of the world’s top ISO and GMP advisers, we provide:
✔ Complete project care from start to finish
✔ Advice tailored to your exact sector
✔ Docs written to fit your way of working
✔ Links to accredited cert bodies
✔ Prep for audits plus follow-up help
We shorten wait times, cut stress and build trust in your products, all at prices that respect your budget.
Ready to Begin Your GMP Certification Journey?
With solid guidance, getting GMP certified stops feeling like a hurdle and starts looking like a fresh growth chance. Popularcert stands ready to walk beside you at every turn.
Claim your FREE chat today and lift your factory process onto a new plane.
GET A FREE CONSULTATION NOW
FAQ
What does GMP mean, and why should anyone care about it?
GMP, short for Good Manufacturing Practices, is a global blueprint that helps factories turn out safe, reliable goods again and again. Following these rules keeps products clean, easy to trace, and friendly to every person who uses them.
Do all businesses really need a GMP certificate, or is that just hype?
GMP paperwork is written into law for drugs, medical gear, and most food plants. In other fields it still matters because retailers, export markets, and even rivals ask for it before signing a deal.
How quickly can Popularcert put a GMP badge on my company?
If your team is mostly ready, Popularcert usually wraps up the process in three to six months. We also offer a fast-track option for urgent projects or brand-new lines.
What will the Popularcert GMP seal cost in a small workshop or a large factory?
Fees differ with size, sector, site, and the specific work involved, so we listen first then spell out a clear, custom quote. With us there truly are no surprise charges.
Can Popularcert support companies outside India or the Middle East?
Absolutely! Popularcert can deliver on-site and remote GMP consulting to customers in Europe, Africa, Southeast Asia, North America, and beyond. Our team knows the key local standards-WHO, US FDA, EU GMP, and more-so you get advice that fits your region.
