GMP Certification: Ensuring Quality and Safety at Every Stage of Production
Introduction:
In a marketplace where a single quality slip can tarnish a brand overnight, having Good Manufacturing Practices, or ISO GMP, stamped on your facility is no longer just a badge to flaunt, it’s a lifeline. Whether you’re turning raw ingredients into baby food, tablets, lotions, or herbal capsules, showing that every step of production is safe, repeatable, and traceable builds instant trust with shoppers and watchdogs.
In the lines that follow, Popularcert-an industry-leading friend for ISO and GMP approvals-walks you through what GMP really is, who absolutely needs it, the many upsides it delivers, and the clean path to getting that coveted certificate.
What Is GMP Certification?
-
Definition and Purpose:
GMP certification proves that your factory habits, equipment, training, and record-keeping work together to turn out products that meet the agreed rules for quality and safety every time. These rules aim to clip away risks in fields like drugs or food so nothing you ship is cross-contaminated, poorly labeled, or harmful to human health. -
ISO and GMP-How They Relate:
Where GMP zooms in on the factory floor, a range of ISO standards either mirror those practices or fill in extra gaps. For example, ISO 22716 guides cosmetic brand owners on GMP-style cleanliness, while ISO 15378 steers plants that make primary packaging for medicines. - Popularcert backs you on both fronts, helping you weave the right ISO and GMP elements into a single, smoother system so you stay compliant at home and market-ready around the globe.
Why GMP Certification Matters
-
Protecting Consumers:
GMP-certified plants work under tight rules and approved routines that stop: -
cross-contamination:
1. product mix-ups hygiene slip-ups unsafe chemicals or germs
2. This cuts health risks and adds trust in your brand. -
Enhancing Product Quality:
Clear paperwork, skilled staff, and spotless rooms let GMP shops turn out cleaner goods with far fewer defects and returns. -
Meeting Regulatory Requirements:
In many countries, GMP is the law-especially for drugs, food, and medical devices. Without it, getting on the shelf can drag on or never happen.
1. The U.S. FDA demands GMP for drugs and dietary supplements.
2. The EU insists on GMP from every supplier in its markets.
3. Several Middle Eastern and Asian nations also need GMP for import permits.
4. Popularcert helps you mesh with these rules worldwide so your site passes audits with ease.
Types Of Certification
- ISO Certification
- ISO 9001 Certification
- ISO 14001 Certification
- ISO 45001 Certification
- ISO 22000 Certification
- ISO 27001 Certification
- ISO 17025 Certification
- ISO 13485 Certification
- ISO 20000-1 Certification
- ISO 22301 Certification
- ISO 50001 Certification
- ISO 37001 Certification
- IATF 16949 Certification
- ISO 29001 Certification
- ISO 31000 Certification
- ISO 20121 Certification
- ISO 10002 Certification
- ISO 41001 Certification
Get Free Consultation
Our Clients


















Key Components of GMP Compliance
GMP rules are broad, yet they rest on five simple pillars you should keep in mind:
-
Cleanliness & Hygiene:
Controlled spaces, protective gear, routine cleaning -
Accurate Documentation:
Logs of production runs, checks, out-of-spec notes, fixes -
Qualified Personnel:
Trained teams, clear duties, ongoing growth -
Equipment Maintenance:
Regular calibration, cleanup, and validation -
Raw Material Controls:
Approved suppliers, easy tracking, suitable storage
When these areas shine, GMP covers every stage-from the first ingredient to the sealed box on a shelf.
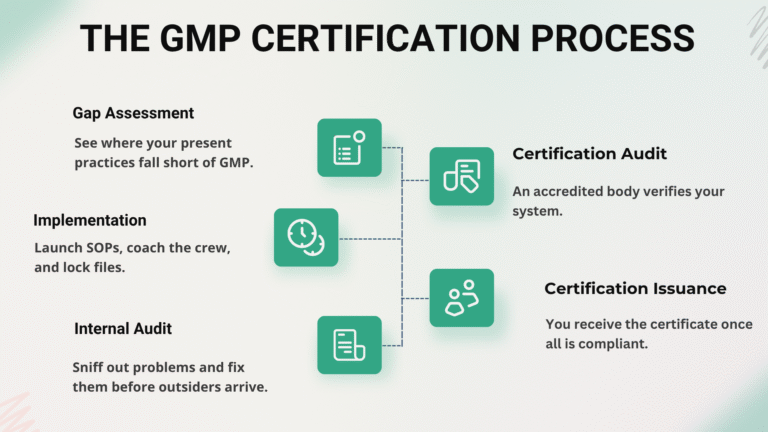
The GMP Certification Process
Securing a formal certificate is not a quick nod; it demands methodical work and trusted counsel.
Step-by-Step Overview:
Timeframe and Costs
How long certification takes depends on your industry, facility size, and the systems you already have in place; many clients finish the process in roughly three to six months. Expenses differ, yet Popularcert offers flexible packages for both start-ups and large plants without cutting corners on audit quality.
Who Needs GMP Certification?
Good Manufacturing Practice (GMP) standards reach a broad range of sectors, such as:
- pharmaceutical makers,
- food and drink suppliers,
- nutraceutical and vitamin firms,
- cosmetic and personal-care brands,
- medical device producers,
- packaging shops that serve pharma and food.
If you make anything people swallow or apply to skin, earning GMP certification safeguards health, wins regulators approval, and earns buyer trust.
Benefits of Getting GMP Certified
- Business advantages
- Brand reputation: protect your name by putting safety and quality first.
- Market expansion: reach regions and clients that insist on GMP (EU, USA, UAE, Oman, and more).
- Supply-chain preference: top the list for global retailers, drugstores, and hospitals.
2. Risk reduction
- Fewer recalls: tight controls and validated processes cut the chance of failures.
- Regulatory protection: dodge fines, product bans, and the scandals they spark..
- Operational efficiency: clear, documented systems trim rework, waste, and costly variations.
How to Prepare for GMP Certification
- The Role of a Consultant
Finding the right GMP guide can make or break your project. That is why companies turn to Popularcert.
Popularcert smooths your journey by delivering:
- Tailored gap checks and road-map plans
- In-person or online training for your crew
- Ready-made docs you can tweak
- Pre-audit coaching and full audit backup
- Help on ISO and GMP so both worlds mesh.
2. Common Pitfalls to Avoid
- Loose docs, missing batch logs, or both
- Staff who have not been trained or briefed
- Messy rooms or layouts that fail the test
- No clear system for fixing faults as they appear
With Popularcert, these snags get spotted early-so audit day feels routine.
Why Choose Popularcert for GMP Certification?
Popularcert is a trusted name worldwide, providing:
- Seasoned experts who have walked your walk
- ISO and GMP steps that fit together naturally
- Full support from first plan to final badge
- Success stories at home and abroad
- Fair rates with no drop-in service.
Whether you operate in Oman, the UAE, India, or farther afield, Popularcert adapts to your market, sector, and maturity. That means you do not just collect a certificate; you secure one with real assurance.
Conclusion
In today’s tight and watchful marketplace GMP certification is far more than a rubber stamp; it is a competitive edge. It proves every batch meets safety, consistency and quality targets, winning respect from regulators and loyal shoppers alike.
Choose Popularcert as your guide and you do not simply tick a compliance box; you gain calm confidence and a stronger place in your industry.
GET A FREE CONSULTATION NOW
FAQs
What’s the difference between GMP and ISO certification?
GMP zeroes in on clean, safe production, while ISO (notably ISO 9001) looks at quality management across the whole business. Because each tackles different but overlapping needs, many firms chase both at the same time.
Is GMP certification mandatory?
In tightly controlled fields such as pharmaceuticals or food manufacturing the answer is yes, the law usually insists on it. Outside those sectors it may not be required, yet most companies still seek it because the endorsement carries serious weight.
How long is GMP certification valid?
Credentials generally last one to three years, then the certifier drops by for an annual check to confirm nothing has slipped out of compliance.
Can small businesses get GMP certified?
Of course they can, and Popularcert teams with start-ups and SMEs to craft lean, budget-friendly road maps that fit limited resources.
