Why Iraqi Companies Should Consider ISO 13485 for Medical Device Quality Management

Introduction: Iraq’s Healthcare Sector on the Rise
Al-Rafidain MedTech is a manufacturer of medical devices in Baghdad who has not been able to meet the qualitative parameters set by both local medical practitioners, as well as buyers across the globe. No doubt, the company is operating in a growing environment. Still, it has thus far shied away from the existing contracts, and has instead relied on the devices which are imported to the country.
Based on the metrics set by the Iraqi Ministry of Health, it is noticeable that over seventy percent of the medical equipment in Iraq is imported, which shows a reliance on external independent institutions. However, local suppliers still hold an opportunity considering the changing landscape in the country, as the investments from the governmental institutions , and the private sector are expected to reach an ascent of eight to ten percent over the coming decade, which is an unprecedented growth. The key would be however, to gain credibility by rapidly ensuring the patient, and the country’s borders are safe, and also align with the ISO 13485 Certification in Iraq which are the international standards to gain.
The Urgency of ISO 13485 Certification
Beyond governance enhancement, Iraq’s health care system continues to be capital oriented reimbursing and expenditure focus which is much more intense on expense reimbursement. Vendors need to balance fiscal profits as well as requests for quotations. It is common to quote foreign examples to justify legislation as the system restarts recovering from stopping attained digitized third-party services to health care and implementing health systems. Social health care is often the case which fractures the populace into different categories of which different sets of remuneration are offered for the purpose of sustaining the system.
Hurting Iraq’s position captures the most recent disadvantages of the country and are embedded into the more overarching strategic frameworks of Iraq’s counterparts as the eroded edges of Iraq’s borders fall into distrust. Calling the system social is too often a cover for a more piracy themed purchase to pay shift. Burden and profit can be amplified and are often influenced by the strategic, tactical and operational decisions made. Competition defined as a paralysis is the most negative influence on technology, and is accompanied by very low marginal subsidies and open tender systems aimed at reconciling a single payer model and the induced selected offers of health care.
Trust is a delicate and precious thing, often formed by purchasing ratios, internal competition and social loops. Predictable, however, in this case, the losing party more often than not is social trust. The system is politically unstable and discontinuous trust is a problem for the automated systems excels in prefinanced mechanisms, the feedback loops are weak. With the exception of margins, social capital and the internal logic defined system drives the results which alone do not require much more breeding behavioral feedback loops.
Overall, Iraq’s healthcare system is a complex interplay of capital management, subsidies, internal competition, and fragile social trust, requiring careful balance between expenditure, reimbursement, and strategic planning.
What is ISO 13485?
ISO 13485 is an international standard concerning quality management systems for medical devices. Although it is more focused than general QMS standards, it still
- Prioritizes patient safety and risk management for each stage of the product life cycle.
- Adheres to legal requirements, both domestic and international.
- Implements systems for traceability and product recall to rapidly rectify account for faulty products.
- Implements ongoing improvement for product quality and operational processes.
In other words, ISO 13485 Certification provides the necessary framework for medical device companies to prove their reliability, safety, and readiness to serve the international market.
How ISO 13485 Supports Iraqi Medical Device Companies
- In the most practical form, the impact of ISO 13485 to Iraqi manufacturers and suppliers are as follows:
- Improves device safety: The devices sold to hospitals are guaranteed to be of high standards.
- Enhances reputation: It builds trust from the regulators in the country and buyers from other nations.
- Creates additional revenue streams: The certification of ISO 13485 is a common requirement needed for both public and private tenders.
- Increases the ease of trade: It serves as an entry point to the GCC, EU, and U.S. markets.
Think about a surgical instrument manufacturer based in Erbil. His devices used to be used only in the country prior to the certification. After certification, identical devices were used in hospitals in Riyadh and Dubai. His case shows how, with the help of ISO 13485, a manufacturer can become a trusted local supplier, then an international one as well.
Top Industries to Choose ISO 13485 in Iraq
All companies in the medical devices sector are subject to ISO 13485. However, a number of industries use this standard more aggressively due to the regulatory scrutiny and the priority given to patient welfare.
- Surgical instruments: Ensures compliance with safety, precision trimming, and sterilization.
- Diagnostic equipment: Guarantees the reliability and accuracy of lab and imaging devices.
- Medical consumables: Ensures hygiene and safety of devices like catheters, syringes, and gloves.
- Dental and orthopedic devices: Ensures safety of prosthetic and implantable products.
- Support devices for hospitals: Ensures the quality of ventilators, infusion pumps, monitors, and other devices.
Focusing on these sectors, Iraqi companies will be able to position themselves as trustworthy partners for hospitals and foreign customers to maximize their relevance in the international market.
Types Of Certification
- ISO Certification
- ISO 9001 Certification
- ISO 14001 Certification
- ISO 45001 Certification
- ISO 22000 Certification
- ISO 27001 Certification
- ISO 17025 Certification
- ISO 13485 Certification
- ISO 20000-1 Certification
- ISO 22301 Certification
- ISO 50001 Certification
- ISO 37001 Certification
- IATF 16949 Certification
- ISO 29001 Certification
- ISO 31000 Certification
- ISO 20121 Certification
- ISO 10002 Certification
- ISO 41001 Certification
Get Free Consultation
Our Clients


















How Popularcert Helps Iraqi Companies Achieve ISO 13485
From experience, understanding the nuances of the ISO 13485 Certification Process can be frustrating, especially for those applying for the certification for the first time. Popularcert has designed a Step by Step approach to make the process easier:
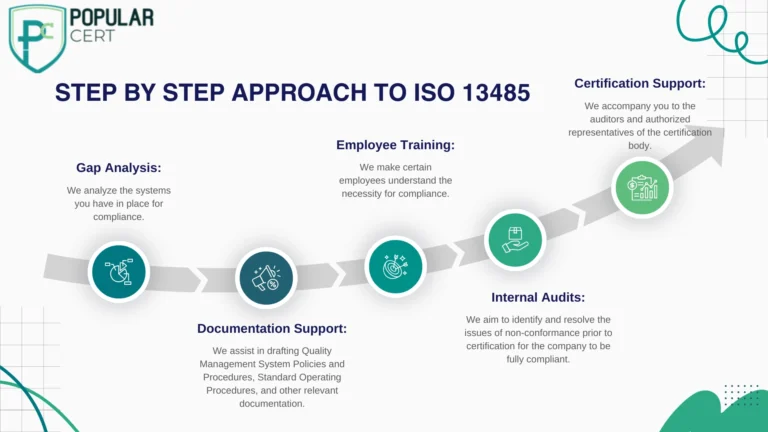
Having specialized services for businesses located in Iraq and a strong presence in the medical device industry, Popularcert has enabled companies such as Al-Rafidain MedTech and Erbil Surgical Instruments to obtain the certification in a timely and cost effective manner while maintaining adherence to the international standards of quality.
Conclusion
Iraqi medical device manufacturing companies will have to stop utilizing informal processes by 2025. ISO 13485 Certification is the bare minimum that needs to be done for safety, maintaining regulations, and competing internationally.
Companies are able to work with expert consultants like Popularcert to gain as much help with documentation, training, and audits as possible. This ensures the strict requirements for local and global market expansion are properly set. In the end, ISO 13485 is more than just a certification. It is the first step toward gaining credibility, growth, and long lasting success in the ever evolving healthcare market in Iraq.
GET A FREE CONSULTATION NOW
FAQs
What is ISO 13485 Certification in Iraq and why is it important?
It is a QMS standard for medical devices that ensures safety, reliability, and compliance with local and international regulations.
Who should get ISO 13485 Certification in Iraq?
Manufacturers, distributors, suppliers, and service providers in the medical device industry.
How does ISO 13485 benefit a medical device QMS in Iraq?
It reduces risks, ensures traceability, enhances operational efficiency, and aligns products with global regulatory requirements.
Can ISO 13485 help Iraqi companies export medical devices?
Yes, certification is often mandatory for GCC, EU, and U.S. markets, which facilitates international expansion.
Why choose Popularcert as ISO 13485 consultants in Iraq?
Popularcert provides comprehensive guidance, documentation support, staff training, and audit assistance to achieve certification smoothly.
