GMP Manufacturing Certification in the Philippines: Your Complete Guide to Compliance & Benefits

Introduction
In the current economy centered around safety and health, obtaining GMP manufacturing Certification In Philippines serves a greater purpose than compliance with regulation—it enhances brand loyalty and trust. In the Philippines, pharmaceutical, food, and cosmetic manufacturers all need to adhere to Good Manufacturing Practices (GMP) for compliance purposes and business advancement.
In this blog, we explore the meaning, advantages, standards, and expert help offered by Popularcert, a worldwide leader in certification consulting, on obtaining a GMP certificate in the Philippines.
What is GMP Manufacturing?
Good Manufacturing Practices (GMP) are a set of regulations, codes, and guidelines pertaining to the manufacturing, testing, and quality assurance processes. The objective of GMP is to ensure products are properly managed and produced as per strict internal company benchmarks and operational quality standards.
- Pharmaceuticals
- Food and beverages
- Nutraceuticals and dietary supplements
- Cosmetics and personal care
- Medical devices
GMP applies to numerous sectors, including:
- GMP is centered on
- Health and sanitation
- Clean environments
- Consistent production techniques
- Documented processes
- Qualified employees and training.
In the Philippines, compliance with GMP is very critical for businesses that need to satisfy the FDA Philippines standards and for those that have intentions to export.
Why is GMP Certification Important in the Philippines?
The Philippines FDA (Food and Drug Administration) imposes the disease controlling authority for good manufacturing practice (GMP) compliance on manufacturers of the health related industries. Absence of this will result in fines, product recalls, and terrible backlash on business image.
Some important aspects as to why GMP is important in Philippines comprise of:
-
Consumer Safety:
Protection from contamination, health adversities, and defects negative health consequences. -
Regulatory Compliance:
Necessary for obtaining license with FDA and export clearance. -
Market Trust:
Enhances credibility for local and international purchasers. -
Export Readiness:
Especially important when entering international markets such as the US, EU, and ASEAN. -
Operational Excellence:
Supports the adoption of effective processes which are documented, well organized, and standardized.
Types Of Certification
- ISO Certification
- ISO 9001 Certification
- ISO 14001 Certification
- ISO 45001 Certification
- ISO 22000 Certification
- ISO 27001 Certification
- ISO 17025 Certification
- ISO 13485 Certification
- ISO 20000-1 Certification
- ISO 22301 Certification
- ISO 50001 Certification
- ISO 37001 Certification
- IATF 16949 Certification
- ISO 29001 Certification
- ISO 31000 Certification
- ISO 20121 Certification
- ISO 10002 Certification
- ISO 41001 Certification
Get Free Consultation
Our Clients


















Who Needs GMP Manufacturing Certification?
GMP is essential or strongly advised for the following businesses in the Philippines:
- Pharmaceutical manufacturers of tablets, syrups, and injections.
- Food manufacturers, processors and packagers.
- Nutraceutical & dietary supplement manufacturers.
- Producers of cosmetic and skincare products.
- Manufacturers of medical devices which are sterile or implantable.
GMP certification is beneficial regardless of whether you are a startup or an established brand because it helps bolster regulatory and quality assurance framework.
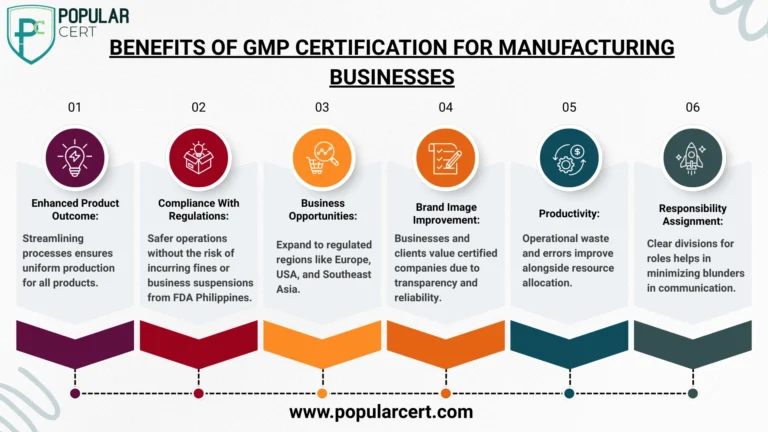
Benefits of GMP Certification for Manufacturing Businesses
Acquiring GMP certification in the Philippines has off-shooting strategic and financial advantages. Here’s what your business can take advantage of:
GMP Standards in Manufacturing: What You Must Follow
Core Requirements of GMP
To remain compliant with best practices, companies have to work within the following areas:
- Hygienic conditions: Cleanliness of the facilities, equipment, and personnel personnel.
- Controlled environments: These are critical for pharmaceuticals and sterile production.
- Standard Operating Procedures (SOPs): There should be clear written steps for every task.
- Qualified personnel: Staff need to be trained and skilled.
- Material traceability: All raw materials and final products need to be documented.
- Equipment calibration and maintenance: This upholds the quality of products made.
- Batch records and documentation: Audits and reviews require everything to be tracked.
Common Issues With the Implementation of GMP
- Steer clear of these issues that may result in you failing certification
- SOPs that are either vague or history based
- Deficient training for personnel
- Lack of proper controlled documentation
- Lack of cleanliness in work areas
- Rare internal auditing
Step-by-Step: How to Get GMP Manufacturing Certification in the Philippines
Achieving GMP Certification can be accomplished in a few steps:
1.Gap Analysis
Evaluate Existing Practices against GMP requirements. Determine what needs to be done and what can be done better.
2. SOPs Creation and Documentation
Develop And document comprehensive Standard Operating Procedures for every process from receiving raw materials to product dispatch.
3. Employe Training
Ensure that your workforce is educated on the SOPs, their role in adherence to GMPs, and the importance of the practices.
4. Facility Preparation рш Preparation of Facilities
Make certain that the specific physical environment/area for work will not fall below cleanliness and operational standards.
5. Internal Audits
Carry out dummy external audits looking for unaddressed gaps so that you can address all of them.
6. Audit from Certification Body
After all internal gaps have been remediated, a third-party to the business will conduct the audit. After that, you will be given a document reporting on the findings.
7. Issuance of Certification and Continued Compliance
Once compliant with the set standards, you will be awarded the GMP certificate. Regular maintenance exercises are to be done for sustaining the certification.
Choosing the Right GMP Consultant in the Philippines
Selecting the right consultant is crucial to avoiding delays and ensuring success.
When choosing a consultant, make sure they:
- Understand FDA Philippines regulations deeply.
- Provide comprehensive services—from documentation to audit assistance.
- Have experience in multiple industries such as food, pharma, and cosmetics.
- Develop training and templates tailored to your needs.
- Assists in maintaining your certification annually.
Why Choose Popularcert for GMP Certification in the Philippines?
Popularcert is an established certification consulting firm with international recognition and a strong foothold in the Philippines. Leading manufacturers prefer Popularcert because:
- Industry Diversity: Popularcert has served clients in pharmaceuticals, food, cosmetics, and supplements.
- Comprehensive Service: Popularcert provides gap analysis, training, and audit preparation.
- Custom Developed Documentation: Includes operation-specific SOPs and other necessary documents.
- Understanding: both Philippine FDA and international standards allow for local and global reach.
- Accelerated Response: Prompt support to facilitate quicker certification.
- Pricing Structure: Competitive with no hidden costs or unreasonable delays.
Start your journey toward trusted, high-quality manufacturing by contacting Popularcert for a free consultation and becoming GMP certified.
Conclusion: Make Your Manufacturing Business Globally Trusted with GMP
For businesses producing food items, cosmetics, or medicines in the Philippines, obtaining a GMP manufacturing certification is critical for safety and compliance. This certification demonstrates your commitment towards quality, increases market access, and ensures national and international legal alignment.
Avoid the pitfalls of delayed or misplaced certifications and consult Popularcert. They specialize in GMP, ISO, and other regulatory certifications, and can assist you in achieving certification quickly.
Reach out today to book a free consultation and begin your path towards compliance and manufacturing excellence.
GET A FREE CONSULTATION NOW
FAQs
Is it compulsory to have GMP certification in the Philippines?
Yes, it is mandatory for food and pharmaceutical manufacturers, as it is an FDA Philippines regulation.
What is the duration for obtaining a GMP certification?
It ranges from one to three months based on the readiness of documents, condition of the facility, and level of compliance among other factors.
Is it possible to use online training for GMP compliance?
That’s acceptable as long as it is from a reputable provider. Popularcert has hybrid options which include partial online sessions.
Are exports certified with GMP more easier to deal with?
Of course. The majority of countries, especially for food and pharmaceuticals, need GMP certification to import products.
What are the consequences if I do not pass the audit for certification?
You will be issued a non-compliance report. Popularcert has strategies to reapply efficiently after resolving issues to help you retake the audit.
