ISO 13485 Certification
Get Free Consultation
ISO 13485 Medical Devices Quality Management System
ISO 13485 is known to be the worldwide standard for any quality management system within the medical device industry, which promotes companies and suppliers involved in manufacturing, supplying, and distributing into meeting strict requirements through regulatory agencies while providing safe and high-quality medical devices. Therefore, risk management, traceability of products, and especially congruence with regulatory requirements come to be essential for all types of healthcare businesses that take part in designing, producing, installing, or servicing medical devices. ISO 13485 certification ensures that a company maintains the best quality management related to regulatory compliance in industries.
PopularCert offers an all-inclusive approach towards ISO 13485 certification, guided by experience and structured implementation to help firms obtain compliance, ensuring better product quality management.
Why Organizations Need ISO 13485?
ISO 13485 ensures that medical device companies adhere to the highest quality and regulatory standards in the industry. Some of the key reasons for pursuing ISO 13485 certification include:
- Regulatory Compliance: The standard helps organizations comply with national and international regulations.
- Product Reliability and Safety: It improves the safety and reliability of medical devices, ensuring better patient outcomes.
- Customer Confidence: Certification boosts customer and stakeholder trust, especially in the healthcare sector.
- Risk Management: Enhances risk management strategies, ensuring a safer product lifecycle.
- Market Expansion: ISO 13485 facilitates access to global markets by meeting necessary regulatory requirements.
How to Get ISO 13485 Certification
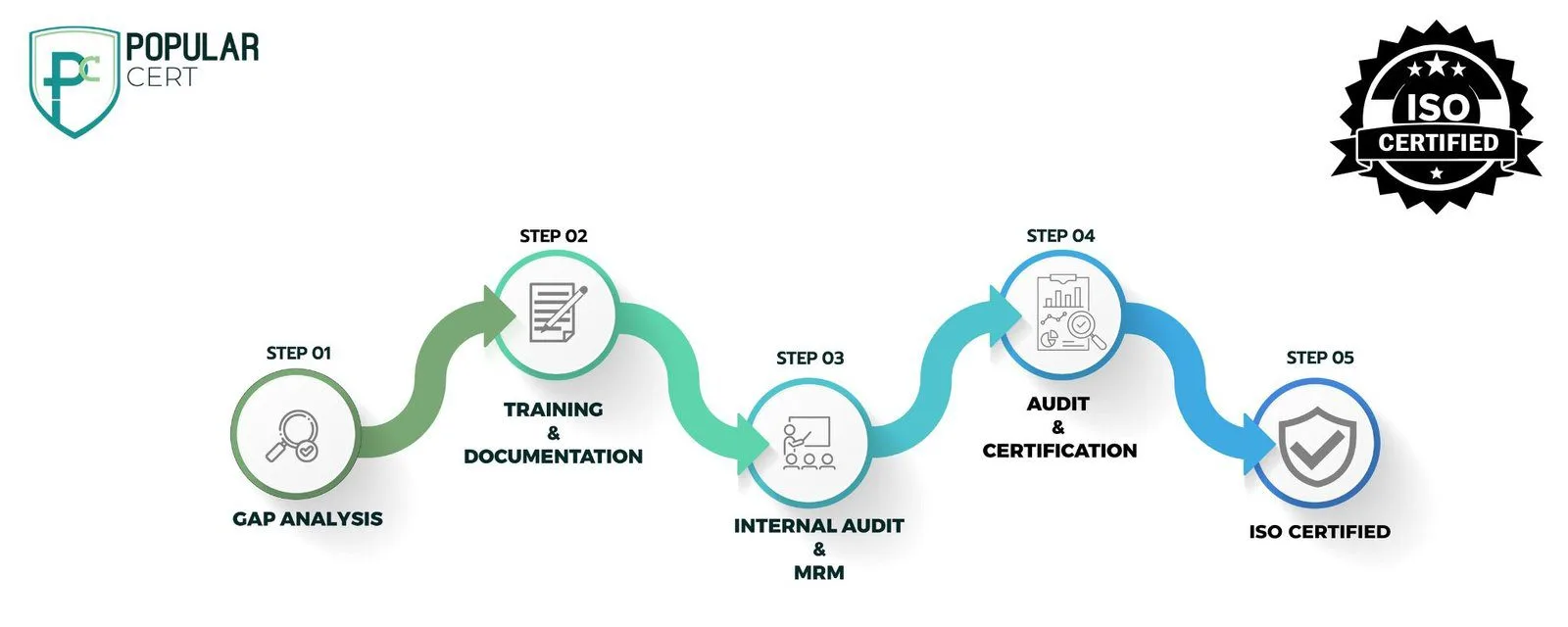
Steps to Achieve ISO 13485 Certification
Gap Analysis & Consultation
Evaluate the current quality management systems against ISO 13485 requirements.
Documentation & Quality Manual Development
Develop the necessary policies, procedures, and quality manuals.
Implementation & Staff Training
rain staff to implement ISO 13485 standards effectively.
Internal Audits & Management Review
Conduct internal audits and management reviews to identify areas of improvement.
Certification Audit & Approval
Undergo an external audit by an accredited certification body for approval.
Principles of ISO 13485 Medical Device QMS:
- Compliance with National and International Regulations: Compliant with both national and international medical device regulations.
- Risk-Based Approach: Concentrates on identifying and managing risks throughout the lifecycle of the product.
- Process Control & Product Safety: Assures traceability, defect prevention, and compliance with safety standards.
- Customer Satisfaction: Focuses on meeting the needs and expectations of healthcare providers and patients.
- Continuous Improvement: Strives for ongoing improvement in quality management processes through continuous monitoring and evaluation.
General Requirements of ISO 13485
When any ISO standard is put into place, some basic needs form the foundation and success of a Quality Management System.
- Standard Compliance: Adhere to all ISO 13485 requirements.
- Documentation & Records: Maintain necessary process documentation and records for compliance.
- Risk Management: Conduct risk assessments and implement mitigation measures.
- Process Standardization: Establish and strictly follow standardized procedures for medical device development.
- Operational Monitoring: Implement tracking mechanisms to monitor activities and correct process deviations.
- Regulatory Compliance: Identify and comply with all relevant legal and regulatory obligations.
- Outsourced Activities: Maintain responsibility for outsourced processes to ensure quality and compliance.
- Validation & Control: Validate manufacturing systems to ensure they function correctly and do not negatively impact operations.
Types Of Certification
- ISO Certification
- ISO 9001 Certification
- ISO 14001 Certification
- ISO 45001 Certification
- ISO 22000 Certification
- ISO 27001 Certification
- ISO 17025 Certification
- ISO 13485 Certification
- ISO 20000-1 Certification
- ISO 22301 Certification
- ISO 50001 Certification
- ISO 37001 Certification
- IATF 16949 Certification
- ISO 29001 Certification
- ISO 31000 Certification
- ISO 20121 Certification
- ISO 10002 Certification
- ISO 41001 Certification
- CE Mark Certification
- Halal Certification
- BIFMA Certification
- RoHS Certification
- HACCP Certification
- GMP Certification
- Organic Certification
- AS9100 Certification
- TL 9000 certification
- SA 8000 certification
- SoC Certification
- GDPR Certification
- HIPAA certification
Get Free Consultation
Our Clients


















Benefits of ISO 13485 Certification
- Industry Regulations Compliance: This ensures that the medical device is compliant with global medical device regulations, especially when entering new markets.
- Improved Product Safety and Quality: It enhances the reliability and safety of medical devices, which are very important to patient well-being.
- Global Market Access: This allows access to international markets and simplifies the process of obtaining regulatory approvals.
- Improved operational efficiency: ISO 13485 helps streamline operations, reducing inefficiencies and possible errors.
- Strengthened Trust: It increases the trust between healthcare providers, patients, and regulatory authorities.
Cost of ISO 13485 Certification
The fee for obtaining certification through ISO 13485 depends on company size, the complexity of manufacturing processes, and the chosen certification body. Expenses usually include consultancy fees, development of documentation, audit costs, employee training, and ongoing maintenance. Obtaining certification will require an initial investment; however, it will bring profitable value in the sense that the product shall be of higher quality, compliant with the regulation, and able to get into international markets.
Why Choose PopularCert for ISO 13485 Certification
PopularCert gives you a convenient way to earn ISO 13485 certification from expert consultants working with customized solutions. Our company covers every single step of the process, meaning that companies end up achieving the compliance efficiently but also improving quality and performance with their products. Whether you have a small start-up or large medical device manufacturers, PopularCert has the help you need for navigating the road to certification.
GET A FREE CONSULTATION NOW
FAQ
Who requires ISO 13485 certification?
Manufacturers, suppliers, and distributors of medical devices who seek to comply with regulatory standards and improve their product quality.
How long does it take to obtain ISO 13485 certification?
Typically take few weeks to Months, depending on the size of the organization and the complexity of their processes.
How does ISO 13485 support regulatory compliance?
ISO 13485 aligns with international medical device regulations, meaning companies are compliant with legal requirements to manufacture, distribute, and have quality control measures in place to gain market access and avoid risks associated with compliance.
What are the key requirements of ISO 13485?
ISO 13485 mandates that an organization develop a documented quality management system, adopt risk management practices, ensure product traceability, perform internal audits, and ensure regulatory compliance at every stage of the medical device lifecycle.
