GMP Certification
Get Free Consultation
What is GMP?
GMP (Good Manufacturing Practice) is an internationally recognized standard for ensuring the safety and quality of products in industries like pharmaceuticals, food, and cosmetics. It provides a structured approach to identifying, preventing, and controlling risks in the manufacturing process. Companies involved in making medicines, food products, cosmetics, and other consumer goods can get GMP certification to show their commitment to high-quality standards and compliance with global safety regulations.
GMP applies to organizations involved in manufacturing, processing, and packaging products that must meet strict quality and safety requirements. It is designed for companies that produce food, pharmaceuticals, cosmetics, and other regulated products, ensuring consistency, hygiene, and continuous improvement in their operations.
Why is GMP Certification Important?
GMP certification is important because it ensures that products are consistently produced and controlled according to high-quality standards. It helps businesses in industries like pharmaceuticals, food, and cosmetics maintain safety, hygiene, and compliance with regulations. By following GMP guidelines, companies reduce risks of contamination, defects, and errors, ensuring that consumers receive safe and reliable products.
Additionally, GMP certification builds customer trust, improves market opportunities, and helps businesses meet legal and international requirements. Many countries and clients prefer to work with GMP-certified companies, making it essential for business growth and global market access.
How to Get GMP Certification
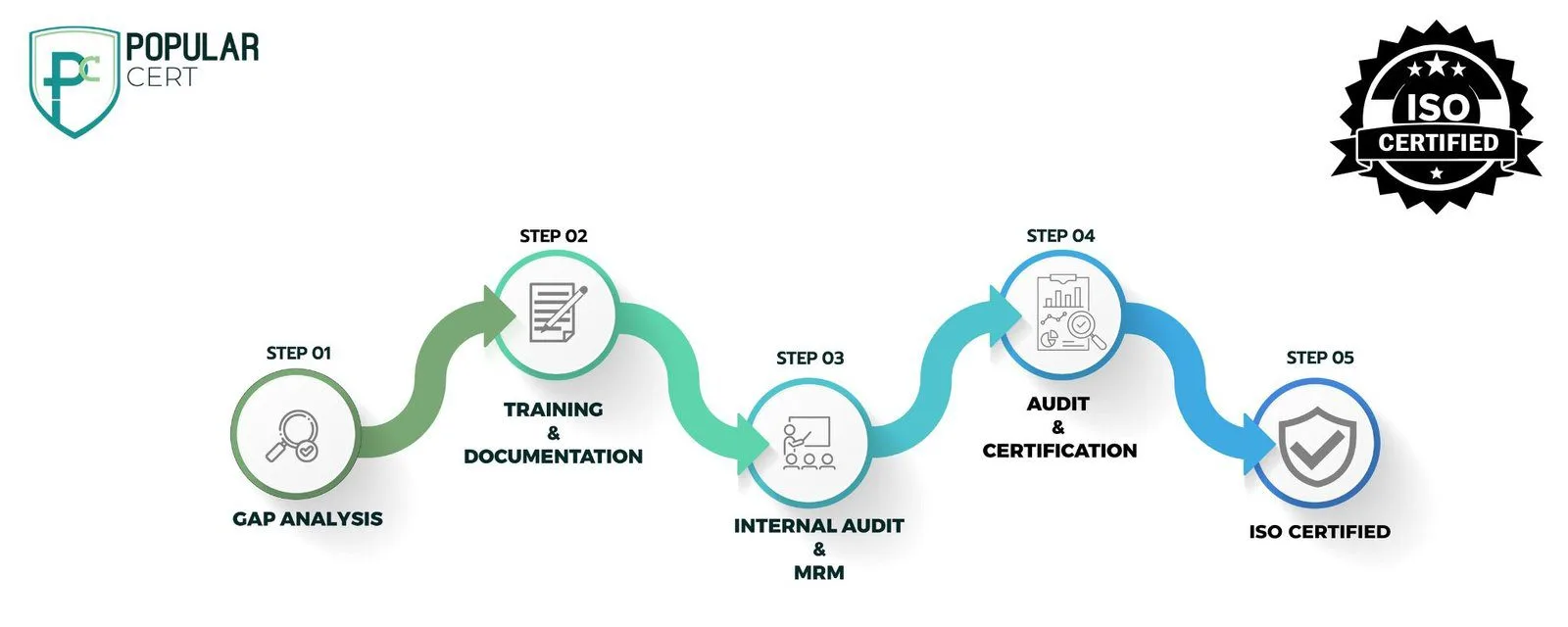
Procedure to Get GMP Certification
Understand GMP Requirements
Learn about GMP guidelines relevant to your industry and ensure your processes align with them.
Conduct a Gap Analysis
Assess your current manufacturing practices and identify areas that need improvement to meet GMP requirements.
Develop Documentation
Prepare standard operating procedures (SOPs), quality policies, and record-keeping systems as required by GMP.
Implement GMP Practices
Apply necessary changes in manufacturing, storage, and quality control processes to meet GMP standards.
Conduct Internal Audits
Perform regular internal audits to check for compliance, identify gaps, and take corrective actions.
Management Review
Hold a review meeting with management to evaluate GMP implementation and address any remaining issues.
Third-Party Audit
Choose an accredited certification body to conduct an external audit and verify compliance with GMP standards.
When to Start Implementing GMP Certification?
The best time to start implementing GMP certification is as soon as your business is ready to improve its quality and safety standards. If you are planning to manufacture food, pharmaceuticals, cosmetics, or other regulated products, it is important to start early. Businesses should begin implementation when they want to ensure product safety, meet regulatory requirements, or expand into new markets. If customers or authorities require GMP compliance, it is essential to start immediately. The sooner you begin, the easier it will be to build a strong quality management system and avoid risks like contamination, recalls, or legal issues.
Benefits of GMP Certification
- Improved Product Quality: Ensures consistent production of high-quality products by following strict standards in manufacturing processes.
- Increased Consumer Safety: Reduces the risk of contamination, defects, and safety issues, ensuring products are safe for consumers.
- Regulatory Compliance: Helps businesses meet local and international regulatory requirements, avoiding penalties and legal issues.
- Enhanced Reputation: Builds consumer trust and strengthens the brand's reputation for producing reliable, safe products.
- Market Access: Opens up new opportunities, as many clients and countries require GMP certification for doing business.
- Continuous Improvement: Encourages ongoing evaluation and improvement of processes, leading to better efficiency and innovation.
- Cost Savings: Reduces product recalls, wastage, and operational disruptions, leading to cost savings in the long term.
Types Of Certification
- ISO Certification
- ISO 9001 Certification
- ISO 14001 Certification
- ISO 45001 Certification
- ISO 22000 Certification
- ISO 27001 Certification
- ISO 17025 Certification
- ISO 13485 Certification
- ISO 20000-1 Certification
- ISO 22301 Certification
- ISO 50001 Certification
- ISO 37001 Certification
- IATF 16949 Certification
- ISO 29001 Certification
- ISO 31000 Certification
- ISO 20121 Certification
- ISO 10002 Certification
- ISO 41001 Certification
- CE Mark Certification
- Halal Certification
- BIFMA Certification
- RoHS Certification
- HACCP Certification
- GMP Certification
- Organic Certification
- AS9100 Certification
- TL 9000 certification
- SA 8000 certification
- SoC Certification
- GDPR Certification
- HIPAA certification
Get Free Consultation
Our Clients


















GMP Internal Audit Checklist
Industries That Benefit from GMP Certification
- Pharmaceutical Industry: Ensures medicines are produced safely, with high quality and consistency.
- Food and Beverage Industry: Guarantees hygienic production, reducing contamination risks in food products.
- Cosmetics Industry: Maintains the safety and quality of skincare, makeup, and personal care products.
- Dietary Supplements Industry: Ensures the purity and effectiveness of vitamins, minerals, and health supplements.
- Biotechnology Industry: Ensures high-quality standards in the development of biological products and research.
- Herbal and Natural Products Industry: Controls quality in herbal medicines, essential oils, and organic health products.
How much does it cost for GMP Certification
The cost of GMP certification can vary based on factors like the size of your business, the complexity of your operations, and where your business is located. Other costs may include gap analysis, system development, employee training, internal audits, and the certification audit itself. There could also be additional costs for ongoing compliance, surveillance audits, and updates to maintain the certification. It’s a good idea to get quotes from a trusted consultant, like PopularCert, to get a more accurate estimate based on your company’s specific needs.
Why choose Popularcert for GMP Certification:
Choosing Popularcert for GMP Certification means you got expert support of a team with over ten years of experience in the certification field. We’ll help you every step of the way, from the initial consultation to getting your certification. Our goal is to help you improve your quality management system, ensure your products meet safety standards, and drive continuous improvement. With over 3,000 successful certifications globally, PopularCert is your trusted partner for achieving GMP certification .
GET A FREE CONSULTATION NOW
FAQ
What industries benefit from GMP certification?
GMP certification is essential for industries like pharmaceuticals, food and beverages, cosmetics, dietary supplements, and medical devices, ensuring product safety, quality, and compliance with regulations.
How long does it take to get GMP certification?
The timeline for GMP certification can vary, but it typically takes around 6 to 12 months, depending on the size of the company, the complexity of operations, and readiness to meet the standards.
What are the main requirements for GMP certification?
GMP certification requires businesses to implement strict quality control procedures, maintain proper documentation, ensure hygiene and safety, and conduct regular internal audits to ensure continuous compliance.
Is GMP certification mandatory?
GMP certification is not legally required by all businesses, but it is often necessary for companies in regulated industries to meet customer demands, comply with international regulations, and improve their market competitiveness.
